Very Short Answer Questions - 1 Mark
Que 1. Name the acid present in vinegar.
Ans. Ethanoic acid
Que 2. Two solutions A and B have pH values of 5 and 8 respectively. Which solution will be basic in nature?
Ans. Solution B
Que 3. If a few drops of a concentrated acid accidentally spills over the hand of a student, what should be done?
Ans. The hand is washed immediately with plenty of water and a paste of sodium hydrogencarbonate (a base) is applied to neutralise the acid.
Que 4. If someone is suffering from the problem of acidity after overeating; which of the following would you suggest as a remedy?
Lemon juice, Baking soda solution or Vinegar.
Ans. Baking soda solution because it is a base and hence neutralises the excess acid present in the stomach.
Que 5. Arrange the following in the increasing order of acidic strength.
Acetic acid, water and hydrochloric acid
Ans. Water < acetic acid < hydrochloric acid.
Que 6. Why does tooth decay start when the pH of mouth is lower than 5.5?
Ans. Tooth starts decaying when the pH of our mouth is lower than 5.5. This is because below this pH value, the medium of the mouth becomes more acidic due to which tooth enamel corrodes at a faster rate.
Que 7. Two solutions X and Y have pH values of 3.0 and 9.5 respectively. Which of these will turn litmus solution from blue to red and which will turn phenolphthalein from colourless to pink?
Ans. X will turn blue litmus to red because it is acidic in nature having pH value less than 7. Y will turn phenolphthalein from colourless to pink because it is basic in nature having pH value greater than 7.
Que 8. A knife, which is used to cut a fruit, was immediately dipped into water containing drops of blue litmus solution. The colour of the solution changes to red, what is the nature of the fruit?
Ans. The Fruit is acidic as it turns blue litmus solution to red.
Que 9. Explain why an aqueous solution of ammonium chloride is acidic in nature.
Ans. Ammonium chloride (NH4Cl) is the salt of a strong acid, hydrochloric acid (HCl), and a weak base ammonium hydroxide (NH4OH), so an aqueous solution of ammonium chloride is acidic in nature.
Que 10. What are the products formed when an acid reacts with a base?
Ans. Acid reacts with a base to form salt and water.
Que 11. Fresh milk has a pH of 6. When it changes into curd (yogurt), will its pH value increase or decrease? Why?
Ans. When milk changes into curd (yogurt), its pH value decreases. This is because during curd formation, lactic acid is produced which makes it acidic.
Que 12. A white chemical compound becomes hard on mixing proper quantity of water. It is also used in surgery to maintain joints in a fixed position. Name the chemical compound.
Ans. Plaster of Paris (Calcium sulphate hemihydrate).
Que 13. Which one of these has a higher concentration of H+ ions?
1M HCl or 1M CH3COOH
Ans. 1M HCl has a higher concentration of H+ ions because it is a strong acid than CH3COOH.
Que 14. In addition to sodium hydrogencarbonate, baking powder contains a substance 'X'. Name the substance 'X'.
Ans. Tartaric acid.
Que 15. What is the commercial name of calcium sulphate hemihydrate?
Ans. Plaster of Paris.
Que 16. Name the substance obtained by the action of chlorine on dry slaked lime.
Ans. Bleaching powder.
Que 17. How many molecules of water of crystallisation are there in
(i) Plaster of Paris (ii) washing soda crystals?
Ans. (i) 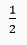
 (ii) 10.
(ii) 10.
Que 18. Why does the milkiness disappear on passing excess of carbon dioxide to lime water?
Ans. When carbon dioxide is passed in excess, calcium carbonate (which appears milky) gets converted into calcium hydrogencarbonate which is soluble. Hence, the milkiness disappears.
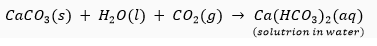
Que 19. An aqueous solution turns red litmus solution blue. Excess addition of which solution would reverse the change-ammonium hydroxide solution or hydrochloric acid?
Ans. hydrochloric acid because adding excess acid to the base would turn blue litmus solution red.
Que 20. Why is the electrolysis of a concentrated solution of sodium chloride known as chlor-alkali process?
Ans. It is because of the products formed: chlor for chlorine and alkali for sodium hydroxide.

