Very Short Answer Questions - 1 Mark
Q. 1. Which subatomic particle is absent in an ordinary hydrogen atom?
Ans. Neutron.
Q. 2. J. Chadwick discovered a subatomic particle which has no charge and has mass nearly equal to that of a proton. Name the particle and give its location in the atom.
Ans. The particle is neutron and it is present in the nucleus of the atom.
Q.3. Is it possible for the atom of an element to have one electron, one proton and no neutron? If so, name the element.
Ans. Yes, it is true for hydrogen atom which is represented as 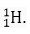
Q. 4. Electron attributes negative charge, protons attribute positive charge. An atom has both but why there is no charge?
Ans. The positive and negative charges of protons and electrons are equal in magnitude. So, atom as a whole is electrically neutral.
Q.5. Write the electronic configuration of an element whose atomic number is 12.
Ans. K, L, M
2, 8, 2
Q. 6. What do you understand by ground state of an atom?
Ans. The state of an atom where all the electrons in the atom are in their lowest energy levels is called the ground state.
Q. 7. What is the maximum number of electrons which can be accommodated in 'N' shell?
Ans. N shell can accommodate maximum 32 electrons.
Q.8. Write the correct representation of an element ‘X' which contains 15 electrons and sixteen neutrons.
Ans. The correct representation of the element X is 
Q. 9. What will be the valency of an atom if it contains 3 protons and 4 neutrons?
Ans. The valency of the atom will be one.
Q. 10. Which of the following pairs are isotopes?
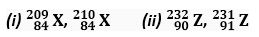
Ans. 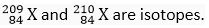
Q. 11. Out of elements  which is chemically more reactive and why?
which is chemically more reactive and why?
Ans. The elements 
Q. 12. One electron is present in the outermost shell of the atom of an element X. What would be the nature and value of charge on the ion formed if this electron is removed from the outermost shell?
Ans. The charge would be + 1.
Q. 13. In the atom of an element X, 6 electrons are present in the outermost shell. If it acquires noble gas configuration by accepting requisite number of electrons, then what would be the charge on the ion so formed?
Ans. - 2.
Q. 14. Give two important applications of radioactive isotopes.
Ans. (i) An isotope of carbon-12, C14, is used in carbon dating.
(ii) U235 is used in the nuclear reactors to generate electricity.
Q. 15. Which isotope of hydrogen is present in heavy water?
Ans. Among the three isotopes of hydrogen, deuterium 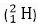
Q. 16. Chemical formula of a metal sulphate is MSO4. What will be the formula of its chloride?
Ans. MCl2
Q. 17. An element 'A' has valency +3, while another element 'B' has valency -2. Give the formula of their compound formed when 'A’ reacts with ‘B'.
Ans. Element 'A' valency +3 (left)
Element 'B' valency - 2 (right)
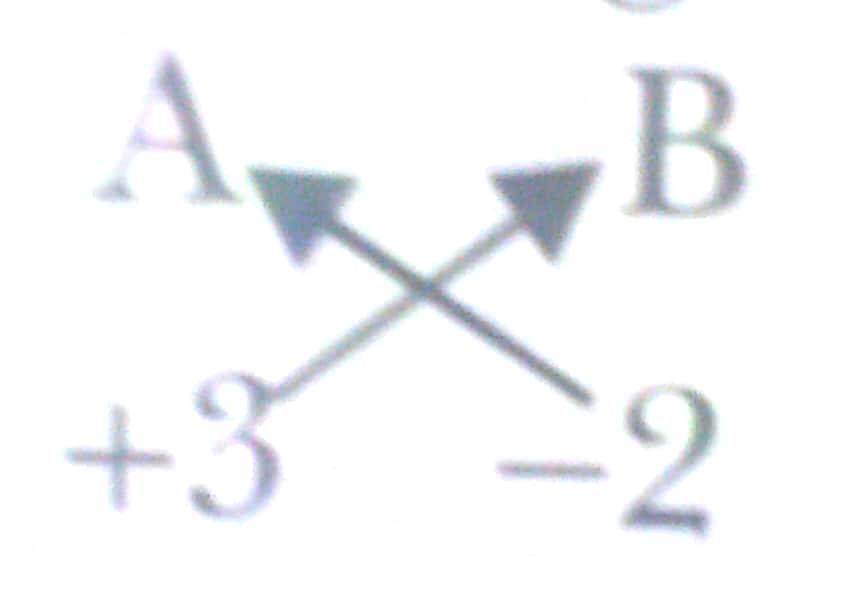
Chemical formula = A2B3
Q. 18. Valency of an element X is 3. Write the chemical formula of its oxide.
Ans. X2O3
Q. 19. Will 35Cl and 37 Cl have different valencies? Justify your answer.
Ans. No, 35Cl and 37Cl are isotopes of an element.
Q. 20. The atomic number of calcium and argon are 20 and 18 respectively, but the mass number of both these elements is 40. What is the name given to such a pair of elements?
Ans. Isobars

