Very Short Answer Questions - 1 Mark
Q. 1. Name two scientists who established the laws of chemical combination?
Ans. Antoine L. Lavoiser and Joseph L. Proust.
Q. 2. Give an example of a triatomic molecule of an element.
Ans. Ozone (O3)
Q.3. Define atomicity.
Ans. It is the number of atoms present in one molecule of a substance.
Q. 4. Write the atomicity of the following molecules:
(i) Sulphur (ii) Phosphorus
Ans. (i) 8 (ii) 4
Q. 5. what is an ion? Give one example.
Ans. The negatively and positively charged particles are called ions.
For example: ![]()
Q.6. Give one word for the following:
(i) A group of atoms carrying a charge
(ii) Positively charged ion
Ans. (i) lon (ii) Cation
Q.7. The atomic number of three elements A, B and C are 9, 10 and 13 respectively. Which of them will form a cation?
Ans. Electronic configuration of A : 2,7
Electronic configuration of B : 2,8
Electronic configuration of C: 2, 8, 3
C" will form a cation because a cation is formed by the loss of one or more electrons by an atom.
Q. 8. What is wrong in saying 'one mole of nitrogen?
Ans. The statement does not clarify whether we are talking about atoms or molecules of nitrogen. We should say 'one mole of nitrogen atoms' or 'one mole of nitrogen molecule’.
Q.9. 'Dalton's atomic theory is contradicted by the formula of sucrose (C12H22O11).'Justify the statement.
Ans. Dalton's atomic theory states that atoms of different elements combine together in simple whole number ratio. In the formula of C12H22O11, the carbon, hydrogen and oxygen combine in whole number ratio but the ratio is not simple.
Q. 10. How many times heavier is one atom of carbon than one atom of oxygen?
Ans. Atomic mass of carbon = 12 u
Atomic mass of oxygen = 16 u
Therefore, one atom of carbon is 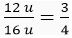 times heavier than one atom of oxygen.
times heavier than one atom of oxygen.

