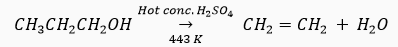Short Answer Questions - I - 2 Marks
Que 1. What is a covalent bond? What type of bond exists in (i) CCl4, (ii) CaCl2?
Ans. The chemical bonds formed between two atoms by the sharing of electrons between them is known as a covalent bond. The sharing of electrons between the two atoms takes place in such a way that both the atoms acquire stable electronic configuration of their nearest noble gas.
(i) CCl4 – Ionic bond –
Ionic bond – Covalent bond, (ii) CaCl2
Covalent bond, (ii) CaCl2
Que 2. Catenation is the ability of an atom to form bonds with other atoms of the same element. It is exhibited by both carbon and silicon. Compare the ability of catenation of the two elements. Give reasons.
Ans. Carbon exhibits catenation much more than silicon or any other element due to its smaller size which makes the C-C bonds strong while the Si -Si bonds are comparatively weaker due to its large size.
Que 3. Select the hydrocarbons which are members of the same homologous series. Give the name of each series.
C3H8, C4H10, C5H10, C6H10, C7H12 and C8H16.
Ans. C3H8, C4H10 → Alkanes
C5H10, C8H16 → Alkanes
C6H10, C7H12 → Alkanes
Que 4. Write the names of the following compounds.
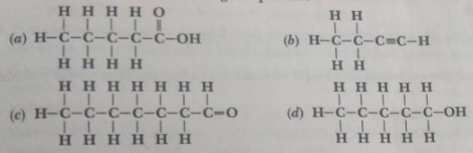
Ans. (a) Pentanoic acid (b) Butyne
(c) Heptanal (d) Pentanol
Que 5. Why are unsaturated hydrocarbons more reactive than saturated hydrocarbons?
Ans. Unsaturated hydrocarbons are more reactive due to the presence of C=C and C=C bonds which are weaker than the single bond in saturated hydrocarbons. These double and triple bonds are the reactive sites in the unsaturated hydrocarbons which easily give addition reactions.
Q. 6. Identify and name the functional groups present in the following compounds.
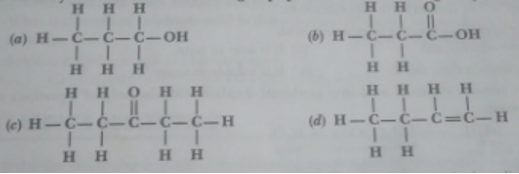
Ans. (a) – OH Hydroxy/Alcohol
OH Hydroxy/Alcohol


Que 7. Write the name and molecular formula of an organic compound having its name suffixed with '-ol' and having two carbon atoms in the molecule. With the help of a balanced equation indicate what happens when it is heated with excess of conc. H2SO4.
Ans. The organic compound is ethanol. Its molecular formula is C2H6O and structural formula is C2H5OH or CH3CH2OH.
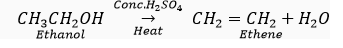
Que 8. Complete the reaction(s) given below and classify them as Combustion/Oxidation/ Addition/Substitution reaction.
(i) 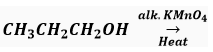
(ii) 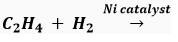
Ans. (i) CH3CH2COOH, Oxidation (ii) C2H6, Addition.
Que 9. Carbon, Group (14) element in the Periodic Table, is known to form compounds with many elements.
Write an example of a compound formed with
(a) Chlorine (Group 17 of Periodic Table)
(b) Oxygen (Group 16 of Periodic Table)
Ans. (a) Carbon tetrachloride (CCL4)
(b) Carbon dioxide (CO2)
Que 10. How is ethanol obtained for commercial use?
Ans. When ethene is heated with concentrated sulphuric acid at 73o C (348 K), and treated with water,ethanol is produced .
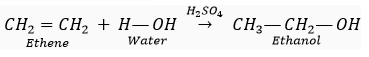
Que 11. Name the gas evolved when ethanoic acid reacts with sodium carbonate. How would you identify this gas?
Ans. The evolved is carbon dioxide (CO2). The reaction is as follows:
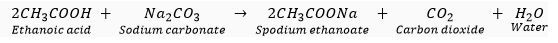
When this gas is passed through lime water, it turns milky. The milky colour of lime water confirms that the gas is carbon dioxide (CO2).
Que 12. Write four uses of ethyl alcohol.
Ans. (i) It is used in the manufacture of paints,medicines, dyes, soaps, etc.
(ii) It is used in the preparation of organic compounds like ether, chloform and iodoform.
(iii) It is used as a fuel in internal combustion engines.
(iv) It is used in low temperature thermometers.
Que 13. Mention the physical properties of acid.
Ans. (i) It is colourless liquid. (ii) It is sour in taste.
(iii) It has a characteristic smell. (iv) It is soluble in water.
Que 14. How will you convert ethanoic acid into methane? Explain with the help of equations of the reactions involved.
Ans. 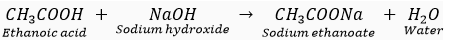
Then, sodium ethanoate is heated with sodalime to get methane.

Que 15. What is meant by denatured alcohol? What is the need to denature alcohol?
Ans. Denatured alcohol is ethyl alcohol which has been made unfit for drinking purposes by adding poisonous substances like methanol, pyridine, copper sulphate, etc.
Ethanol is an important chemical. It is supplied at concessional rates to industries. It is therefore, made unfit for drinking purposes to prevent its misuse.
Que 16. Intake of small quantity of methanol can be lethal. Comment.
Ans. Methanol is oxidised to methanal in the liver. Methanal reacts rapidly with the components of cells. It causes the protoplasm to coagulate. It also affects the optic nerve, causes blindness.
Que 17.A gas is evolved when ethanol reacts with sodium. Name the gas evolved and also write the balanced chemical equation of the reaction involved.
Ans. Gas evolved is hydrogen.
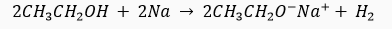
Que 18. Why are detergents better cleansing agents than soaps?
Ans. Detergents work as cleansing agent in hard and soft water both because the charged ends of detergents do not form insoluble precipitates with calcium and magnesium ions in hard water.
Que 19. Why are soaps not suitable for washing clothes with hard water?
Ans. Soaps are not suitable for washing clothes with hard water because of two reasons:
(i) Soap reacts with the calcium and magnesium ions present in hard water to form insoluble precipitate called scum. This results in the wastage of soap.
(ii) The sticky scum sticks to the clothes being washed and interferes with the cleaning ability of soap. This makes the cleaning of clothes difficult.
Que 20. Two carbon compounds A and B have the molecular formula C3H8 and C3H6 respectively. Which one of the two is most likely to show addition reaction? Justify your answer.
Ans. Compound A (C3H8) is saturated and compound B (C3H6) is unsaturated hydrocarbon (with a double bond). As we know that addition reactions are a characteristic property of unsaturated hydrocarbons, thus the compounds B (C3H6) is most likely to show addition reaction.
Que 21. How would you bring about the following conversions? Name the process and write the reaction involved.
(a) Ethanol to ethene. (b) Propanol to propanoic acid.
Ans. (a) By the dehydration of ethanol in the presence of concentrated H2SO4.
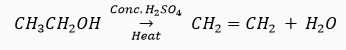
(b) By the oxidation of propanol using oxidising agent such as alkaline KMnO4.
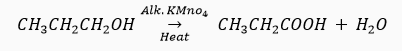
Que 22. Ethene is formed when ethanol at 443 K is heated with excess of concentrated sulphuric acid. What is the role of sulphuric acid in this reaction? Write the balanced chemical equation of this reaction.
Ans. Sulphuric acid acts as a dehydrating agent.