Very Short Answer Questions - 1 Mark
Que 1. Why are metals good conductors of electricity?
Ans. Metals are good conductors of electricity because they contain free electrons. These free electrons move easily through the metal and conduct electric current.
Que 2. Which property of graphite is utilised in making electrodes?
Ans. Graphite is a good conductor of electricity. Due to this property, graphite is utilized in making electrodes.
Que 3. Which of the following metals will melt at body temperature?
Gallium, Magnesium, Caesium, Aluminium
Ans. Gallium and caesium will melt at 37°C (body temperature).
Que 4. Name two metals that do not react with water at all.
Ans. Lead and copper.
Que 5. What happens when calcium is treated with water?
Ans. Calcium reacts less violently with water and bubbles of hydrogen gas stick to its surface.
Que 6. Generally, non-metals are not lustrous. Name a non-metal which is lustrous.
Ans. Iodine
Que 7. What is the nature of non-metal oxide?
Ans. Non-metal oxides are acidic or neutral in nature.
Que 8. What is the nature of metal oxides?
Ans. Metal oxides are basic in nature.
Que 9. Why do copper objects develop a green coating in air?
Ans. Copper reacts with moist carbon dioxide in the air and gains a green coating of basic copper carbonate.
Que 10. Why do silver articles become black on prolonged exposure to air?
Ans. Sulphur compounds such as hydrogen sulphide gas (H2S) present in the air when combine with the silver articles, form a black coating of silver sulphide (Ag2S).
Que 11. Which oxide of iron could be obtained on prolonged reaction of iron with steam?
Ans. Fe3O4
3Fe(s) + 4H2O (g)
Que 12. Why are ionic compounds usually hard?
Ans. In all ionic compounds, their positive and negative ions are attached to each other by a strong ionic bond. So, they are rigid and hard solids.
Que 13. Why does aluminium not react with water under ordinary conditions?
Ans. Aluminium does not react with water under ordinary conditions because of the presence of a thin layer of aluminium oxide on its surface.
Que 14. In nature, metal A is found in a free state while metal B is found in the form of its compounds. Which of these two will be nearer to the top of the activity series of metals?
Ans. Metal B will be nearer to the top of the activity series of metals as it is so reactive that it is found in combined state.
Que 15. Arrange the following metals in decreasing order of their reactivity:
Fe, Zn, Na, Cu, Ag
Ans. Na > Zn > Fe> Cu >Ag.
Que 16. Why cannot aluminium be obtained by reduction of its oxide with carbon?
Ans. Aluminium has more affinity for oxygen than carbon.
Que 17. Why does a little addition of carbon in iron make it more useful?
Ans. Pure iron is very soft and stretches easily when hot. When it is mixed with a small quantity of carbon (0.05%), it becomes hard and strong and hence becomes more useful.
Que 18. Give an example of a sulphide ore which is reduced to metal by heating alone, i.e., by roasting.
Ans. Cinnabar (HgS) on roasting is first changed to mercuric oxide which on further heating is reduced to mercury.
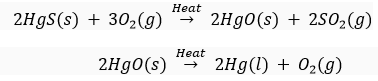
Que 19. Metals are refined by using different methods. Name two metals refined by electrolytic refining.
Ans. Copper and gold.
Que 20. What is rust?
Ans. The coating of brown, flaky substance on the surface of iron when it is kept exposed in moist air is called rust.
Que 21. What is corrosion?
Ans. When the surface of a metal is attacked by air, water and some other substances, it is said to be corroded. This phenomenon is known as corrosion.
Que 22. What is aqua regia?
Ans. It is a freshly prepared mixture of concentrated hydrochloric acid and concentrated nitric acid in the ratio of 3: 1. It can dissolve gold. It is a highly corrosive liquid.
Que 23. Which metals are mixed with iron to get stainless steel?
Ans. Nickel and chromium.
Que 24. Why is stainless steel preferred for making household utensils?
Ans. Stainless steel is preferred as it is non-reactive and so the milk or food is not spoiled in it.
Que 25. What is galvanisation?
Ans. Galvanisation is a method of protecting steel and iron from rusting by coating them with a thin layer of zinc.
Que 26. Name an alloy of
(i) aluminium used in the construction of aircraft.
(ii) lead used in joining metals for electrical work.
Ans. (i) Duralium (ii) Solder.

