Short Answer Questions - I - 2 Marks
Que 1. Which among the following are physical or chemical changes?
(a) Evaporation of petrol
(b) Burning of Liquefied Petroleum Gas (LPG)
(c) Heating of an iron rod to red hot
(d) Curdling of milk
(e) Sublimation of solid ammonium chloride
Ans.
(a) Physical change
(b) Chemical change
(c) Physical change
(d) Chemical change
(e) Physical change
Que 2. How do we come to know that a chemical reaction has taken place?
Ans. The presence of any of the following changes helps us to determine that a chemical reaction has taken place.
(i) Formation of new substance(s)
(ii) Change in state
(iii) Change in colour
(iv) Change in temperature
(v) Formation of a precipitate
(vi) Evolution of a gas
For example, if on mixing two substances a gas is evolved, then we can say that a chemical reaction has taken place.
Que 3. What is an oxidation reaction? Give an example of oxidation reaction. Is oxidation an exothermic or an endothermic reaction?
Ans. The reaction in which oxygen combines with other elements or compounds is known as an oxidation reaction. For example, burning of hydrogen is an oxidation process in which hydrogen combines with oxygen to form water.
2H2(g) + O2(g) → 2H2O(l)
Oxidation reactions are exothermic.
Que 4. Why do fire flies glow at night?
Ans. Fire flies have a protein which in the presence of an enzyme undergoes aerial. This is a chemical reaction which involves emission of visible light. Therefore, fire files glow at night.
Que 5. Give reasons:
(a) Aluminium is a reactive metal but is still used for packing food articles.
(b) Red litmus paper turns blue when touched with aqueous solution of magnesium oxide.
Ans. (a) On exposure to air, aluminium forms a hard protective layer of aluminium oxide (Al2O3) which prevent further oxidation.
(b) Magnesium oxide is an oxide of a metal, so, it is basic in nature. Due to its basic character it turns red litmus paper blue when touched with its aqueous solution.
Que 6. What happens when silver chloride is exposed to sunlight? Write a chemical equation for this reaction. Also give one use of such a reaction.
Ans. When silver chloride is exposed to light, it decomposes to form silver metal and chlorine gas.
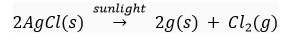
This reaction is used in black and white photography.
Que 7. What type of chemical reactions are represented by the following equations?
(i) A + B
(iii) A
Ans. (i) Combination reaction (ii) Displacement reaction
(iii) Decomposition reaction (iv) Double displacement reaction
Que 8 Complete the missing components/variables given as a and y in the following reactions:
(i) Pb(NO3)2(aq) + 2KI(aq)
(ii) Cu(s) + 2AgNO3(aq)
(iii) Zn(s) + H2SO4(ag)
(iv) 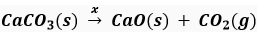
Ans. (i) x
(ii) x
(iii) x
(iv) x
Que 9 Zinc liberates hydrogen gas when reacted with dilute hydrochloric acid, whereas copper does not. Explain why?
Ans. Zinc is above hydrogen whereas copper is below hydrogen in the activity series of metals. That is why zinc displaces hydrogen from dilute hydrochloric acid, while copper does not.
Zn + HCl  2 + H2ZnCl
2 + H2ZnCl
Cu + HCl No reaction
No reaction
Que 10. On adding dilute HCI to copper oxide powder, the solution formed is blue- green. Predict the new compound formed which imparts a blue-green colour to the solution.
Ans. The new compound formed is copper(II) chloride (CuCl2), which imparts blue-green colour to the solution.
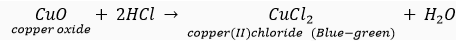
Que 11. Ferrous sulphate decomposes with the evolution of a gas having a characteristic odour of burning sulphur. Write the chemical reaction involved and identify the type of reaction.
Ans. 
It is a thermal decomposition reaction.
Que 12. Identify the substance oxidised, substance reduced, oxidising agent and reducing agent:
MnO2 + 4HCl
Ans.
Substance oxidized : HCI
Substance reduced : MnO2
Oxidising agent : MnO2
Reducing agent : HCI
Que 13. Grapes hanging on the plant do not ferment but after being plucked from the plant can be fermented. Under what conditions do these grapes ferment? Is it a chemical or a physical change?
Ans. Grapes when attached to the plants are living and therefore, their own immune system prevents fermentation. The microbes can grow in the plucked grapes and under anaerobic conditions these can be fermented. This is a chemical change.
Que 14. A copper coin was kept dipped in silver nitrate solution for a few hours/days. What will happen to the copper coin? What will happen to the colour of the solution?
Ans. Copper is more reactive than silver. Hence, it displaces silver from the silver nitrate solution according to the given reaction.
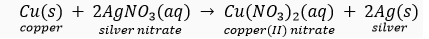
The silver thus formed is deposited on the surface of copper, thereby giving it a white shining appearance.
The solution becomes blue due to the formation of copper nitrate.
Que 15. Identify the reducing agent in the following reactions.
(a) 4NH3 + 502
(b) H2O + F2
(c) Fe2O3 + 3CO
(d) 2H2 + O2
Ans. (a) Ammonia (NH3)
(b) Water (H2O) as F2 is getting reduced to HF
(c) Carbon monoxide (CO)
(d) Hydrogen (H2)
Que 16. What is the role of a catalyst in a chemical reaction?
Ans. Catalyst changes (usually increases but sometimes decreases) the rate of a chemical reaction without itself being consumed in the reaction.

