Very Short Answer Questions - 1 Mark
Q.1. Is There any similarity in materials ?
Ans. Yes, all materials possess mass and occupy space.
Q. 2. When 50 g of sugar is dissolved in 100 mL of water, there is no increase in volume. What characteristic of matter is illustrated by this observation?
Ans. This observation indicates that particles of water have spaces between them into which sugar Particles fit.
Q. 3. What happens when an inflated air balloon is pricked with a pin? Name the property of the gaseous state exhibited by this observation.
Ans. The balloon bursts and diffusion takes place.
Q. 4. Name the process which occurs when a drop of dettol is added to water.
Ans. When dettol is added to water, diffusion takes place.
Q. 5. To which physical state of matter do the following statements apply?
(i) Incompressible, no fixed shape
(ii) Compressible, no definite volume
Ans. (i) Liquid (ii) Gas
Q.6. Name the state of matter in which:
(i) Layers of particles can slip and slide over one another easily.
(ii) Particles just move around randomly because of very weak force of attraction.
Ans. (i) Liquid state, (ii) Gaseous state.
Q. 7. Define density and give its SI unit.
Ans. Density of a substance is defined as the mass per unit volume. Its SI unit is kgm-3
Q.8. In which of the following, the particles have highest forces of attraction?
Water, NaCI (solid), ice or, wax.
Ans. NaCl (solid) has particles with the highest forces of attraction
Q.9. Why do the gases exert more pressure on the Wall of the wall of the container than the solids?
Ans. In gases, the particles move randomly at high speed and they collide with each other and with the container .
Q. 10. Which of the following diffuses faster?
Water vapour, wax or, ethyl alcohol.
Ans. Water vapour
Q. 11. Why do we see water droplets on the outer surface of a glass containing ice cold water?
Ans. The water vapour present in the air comes in contact with cold surface of the glass, loses its energy and gets converted into droplets of water.
Q. 12. Can materials exist in all the three states?
Ans. Yes, materials can exist in all the three states under different conditions of temperature and pressure.
Q. 13. Kinetic energy of particles of water in three vessels A, B and C are EA, EB and Ec respectively and EA> EB>EC Arrange the temperatures, TA, TB and Tc of water in the three vessels in increasing order.
Ans. Tc < TB < TA, the kinetic energy of particles is greater at higher temperature.
Q. 14. Analyse the temperature versus time graph of water, given below.
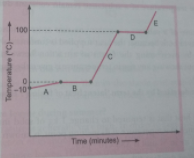
Which region contains all liquids?
Ans. Region C

