Short Answer Questions - II - 3 Marks
Que 1. When zinc metal treated with a dilute solution of a strong acid, a gas is evolved, which is utilised in the hydrogenation of oil. Name the gas evolved. Write the chemical equation of the reaction and also write a test to detect the gas formed.
Ans. When zinc reacts with dilute solution of strong acid, it forms salt and hydrogen gas is evolved.
Zn + 2HCl
When a burning splinter is brought near the mouth of the test tube, the gas burns with a pop sound.
Que 2. Which acid is produced in our stomach? What happens if there is an excess of acid in the stomach? How can its effect be cured?
Ans. hydrochloric acid is produced in our stomach. The excess acid in the stomach causes indigestion which produces pain and irritation. This effect can be cured by using antacids. Antacids (a group of mild bases) react with excess acid in the stomach and neutralise it.
Que 3. To the three solutions listed below, a few drops of phenolphthalein and blue litmus were added separately. Specify the colour change in each case, if any:
|
Name of the solution |
Colour change with phenolphthalein |
Colour change with blue litmus |
|
(a) Sodium carbonate (b) Hydrochloric acid (c) Sodium chloride |
|
|
Ans.
|
Name of the solution |
Colour change with phenolphthalein |
Colour change with blue litmus |
|
(a) Sodium carbonate (b) Hydrochloric acid (c) Sodium chloride |
Turns pink No change No change |
No change Turns red No change |
Que 4. A salt X when dissolves in distilled water gives a clear solution which turns red litmus blue. Explain this phenomenon.
Ans. Basic solution turn red litmus paper blue. The salt of a weak acid and a strong base gives a basic solution. So, the gives salt X is the salt of a weak acid and a strong base.
Example: When sodium carbonate is dissolved in water, it gets hydrolysed to some extent and forms sodium hydroxide and carbonic acid.

Being a strong base, sodium hydroxide is fully ionised and gives a large amount of hydroxide ions (OH-). Carbonic acid is a weak acid which is only slightly ionised and hence, gives a small amount of hydrogen ions (H+). The H+ ions produced by carbonic acid neutralises only a small amount of OH- ions produced by sodium hydroxide and the rest amount of OH- ions are present in the solution. Hence, the Na2CO3 solution is basic in nature. It turns red litmus blue.
Que 5. With the help of a chemical equation, explain how a soda-acid fire extinguisher helps in putting out a fire.
Ans. Soda-acid fire extinguisher contains sodium bicarbonate and sulphuric acid, which are in separate containers in them. When knob of the fire extinguisher is pressed, then sulphuric acid mixes with sodium bicarbonate solution and produces a lot of CO2 gas, which forms a blanket over the fire and cuts it off from the supply of the air to the burning substance and the fire stops.
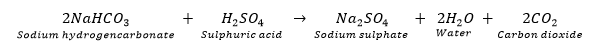
Que 6. A compound which is prepared from gypsum has the property of hardening when mixed with a proper quantity of water. Identify the compound. Write the chemical equation for its preparation. For what purpose is it used in hospitals?
Ans. The compound prepared from gypsum on heating it at 100°C, is known as Plaster of Paris. Its chemical formula is 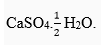 Hence, its chemical name is calcium sulphate hemihydrate. The chemical equation for its preparation is as follows:
Hence, its chemical name is calcium sulphate hemihydrate. The chemical equation for its preparation is as follows:
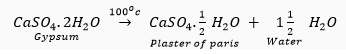
Plaster of Paris is used in hospitals mainly as plaster for supporting fractured bones in the right position. In dentistry, it is used for making casts.
Que 7. What is meant by water of crystallisation? Explain that the crystallisation salts contain water of crystallisation.
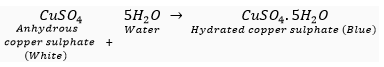
Ans. Water of crystallisation is a fixed number of water molecular present in one formulas unit of a salt. One formula unit of copper sulphate contains five water molecules (5H2O). The water molecules which form part of the structure of a crystal are called water of crystallisation. When hydrated salts are heated strongly, they lose their water of crystallisation.
On strong heating, blue copper sulphate turn white (due to the loss of water of crystallisation).

Anhydrous copper sulphate turns blue on adding water.
Que 8. (i) Write the formula and chemical name of bleaching powder.
(ii) Write chemical equation to represent the action of atmospheric CO2 gas on bleaching powder when left exposed in open.
(iii) State for what purpose is bleaching powder used in water treatment plants.
Ans. (i) Chemical formula: CaOCl2
Chemical name: Calcium oxychloride
(ii) 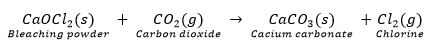
(iii) Bleaching powder is used in water treatment plants for disinfecting drinking water to make it free of germs.
Que 9. How would you distinguish between baking powder and washing soda by heating?
Ans. The chemical formula of backing powder is sodium hydrogencarbonate (NaHCO3); whereas, that of washing soda is sodium carbonate (Na2CO3.10H2O).
Sodium hydrogencarbonate on heating gives CO2gas which will turn lime water milky whereas no such gas is obtained from sodium carbonate.
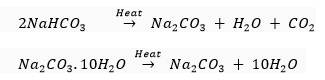
Que 10. Salt A commonly used in bakery products on heating gets converted into another salt B which itself is used for removal of hardness of water and a gas C is evolved. The gas C when passed through lime water, turns it milky. Identify A, B and C.
Ans. Baking powder (NaHCO3), salt A is commonly used in bakery products. On heating, it forms sodium carbonate (Na2CO3), B and CO2 gas, C is evolved. When CO2 gas is passed through lime water it forms calcium carbonate (CaCO3), which is slightly soluble in water making it milky.
A
Que 11. What are strong and weak acids? In the following list of acids, separate strong acids weak acids: Hydrochloric acid, citric acid, acetic acid, nitric acid, formic acid, sulphuric acid.
Ans. In aqueous solutions, strong acids ionise completely and provide hydronium ions. On the other hand, weak acids are partially ionised and an aqueous solution of same molar concentration provides a much smaller concentration of H3O+ ions.
Strong acids
Weak acids
Que 12. What happens when dilute hydrochloric acid is added to the following and write balanced chemical equations
(i) Bleaching powder
(ii) Zinc granules
(iii) Baking soda
Ans. (i) Calcium chloride and chlorine gas are formed.
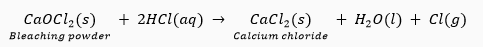
(ii) Hydrogen gas is evolved.
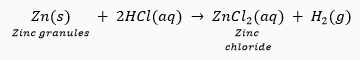
(iii) Sodium chloride and carbon dioxide gas are formed.
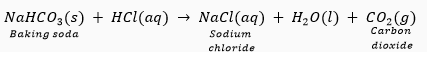
Que 13. Give suitable reason for the following statements:
(i) We feel burning sensation in the stomach when we overeat.
(ii) The crystals of washing soda change to white powder on exposure to air.
(ii) An aqueous solution of sodium chloride is neutral but an aqueous solution of sodium carbonate is basic.
Ans. (i) when we overeat excess of acid is produced in the stomach which causes burning sensation.
(ii) Washing soda is sodium carbonate decahydrate which when exposed to air loses 10 molecules of water and changes to white powder.
(iii) Sodium chloride is a salt of strong acid HCl and strong base NaOH, so it is neutral. Sodium carbonate is a salt of weak acid H2CO3, and strong base NaOH, so it is basic.

