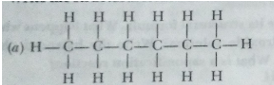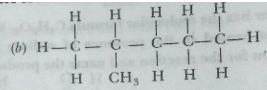Short Answer Questions - II - 3 Marks
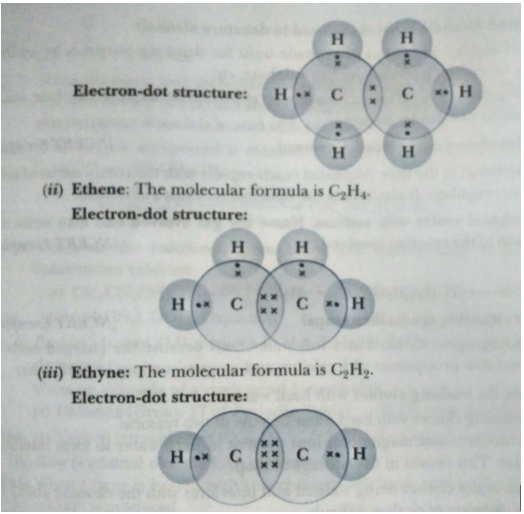
Que 1. Write the molecular formula of the following compounds and draw their electron-dot structures:
(i) Ethane (ii) Ethene (ii) Ethyne
Ans. (i) Ethane: The molecular formula is C2H6.
Que 2. What is meant by functional group in carbon compounds? Write in tabular form the structural formula and the functional group present in the following compounds:
(i) Ethanol (i) Ethanoic acid
Ans. An atom or a group of atoms which determine the chemical properties of an organic compound is called a functional group.
|
Name of compound |
Structural Formula |
Functional |
|
|
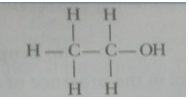 |
 |
|
|
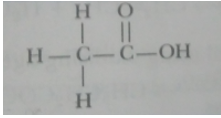 |
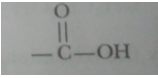 |
Que 3. Draw the electron-dot structure for ethyne. A mixture of ethyne and oxygen is burnt for welding. In your opinion, why cannot we use a mixture of ethyne and air for this purpose?
Ans. For structure of ethyne, Refer to Q. 1, short Answer Questions-11.
Ethyne burns in air with a sooty flame because of incomplete combustion caused by the limited supply of air. But when burnt at 3000oC in oxygen it gives a clean flame because of complete combustion. This oxy-acetylene flame is used for welding.
Such a high temperature cannot be achieved without mixing oxygen.
Therefore, a mixture of ethyne and air is not used for welding.
Que 4. What is meant by isomers? Draw the structures of two isomers of butane, C4H10. Explain why we cannot have isomers of first three members of alkane series.
Ans. Isomers are compounds having the same molecular formula but different structures.
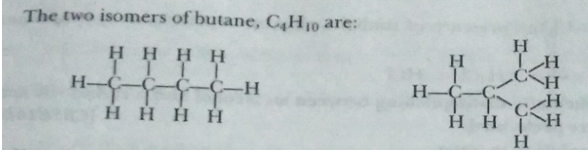
Since branching is not possible, isomers are not possible for the first three members of alkane series.
Que 5. What is meant by homologous series of carbon compounds? Classify the following carbon compounds into two homologous series and name them.
C3H4, C3H6, C4H6, C4H8, C5H8, C5H10
Ans. A group of organic compounds having the same functional group and similar structures in which the successive members differ by CH2 group is called homologous series.
Alkynes: C3H4, C4H6, C5H8
Alkenes: C3H6, C4H8, C5H10
Que 6. Give an example each of (i) open chain (ii) branched chain and (iii) ring compounds.
Ans. (i) Open chain compound (n-pentane):
CH3  CH2
CH2  CH2
CH2  CH2
CH2  CH3
CH3
(ii) Branched chain compound (isobutane):
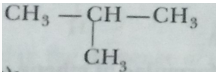
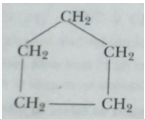
(ii) Ring (cyclopentane):
Que 7. (a) How does the supply of air affect combustion of saturated hydrocarbons?
(b) What is indicated by:
(i) sooty flame (ii) blue flame of a bunsen burner?
(c) Why are holes provided at the bottom of a bunsen burner?
Ans. (a) If air supply is not sufficient, saturated hydrocarbons give yellow sooty flame.
(b) (i) Yellow sooty flame-Burning of unsaturated hydrocarbons such as ethene and ethyne/incomplete combustion (any one),
(ii) Blue flame-Burning of saturated hydrocarbons/complete combustion.
(c) Holes let the supply of air to be adjusted for complete combustion.
Que 8. Explain the given reactions with examples:
(a) Combustion reaction
(b) Oxidation reaction
(c) Substitution reaction
Ans. (a) Combustion reaction: Carbon compounds burns in oxygen to produce carbon dioxide along with release of large amount of heat and light.
CH4 + 2O2
(b) Oxidation reaction: Ethanol is oxidised to ethanoic acid in the presence of alkaline KMnO4 (oxidising agent) on heating.
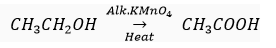
(c) Substitution reaction: In the presence of sunlight, chlorine replace the hydrogen atom of hydrocarbons.
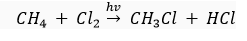
Que 9. List two tests for experimentally distinguishing between an alcohol and a carboxylic acid and describe how these tests are performed.
Ans. (i) Test with NaHCO3 solution in water.
On adding carboxylic acid to baking soda, carbon dioxide is liberated with brisk effervescence.
On adding a solution of baking soda to alcohol, no brisk effervescence occurs.
(ii) Test with blue litmus solution.
Carboxylic acid turns blue litmus red.
There is no change in colour when a blue litmus solution is added to alcohol.
Que 10. Write physical properties of ethanol.
Ans. Physical properties of ethanol are:
(i) Ethanol is a colourless liquid having a pleasant smell and a burning taste.
(ii) Ethanol is a liquid at room temperature.
(iii) Ethanol is lighter than air.
(iv) Ethanol is miscible with water.
(v) Ethanol is a covalent compound.
(vi) Ethanol has no effect on litmus solution.
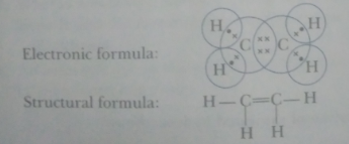
Que 11. Write molecular, electronic and structural formulae of ethene.
Ans. Molecular formula: C2H4
Electronic formula:
Structural formula:
Que 12. (i) What is a homologous series of organic compounds? State any two characteristics of homologous series.
(ii) Draw the electron dot structure for propanal.
Ans. (i) A series of compounds in which the same functional group substitutes for hydrogen in a carbon chain is called homologous series.
Characteristics of homologous series:
(a) The molecular formulae of any two successive members of a homologous series differ by
(b) There is a regular gradation in physical properties of members of a homologous series.
(ii) Propanal (CH3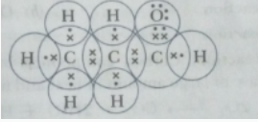
Que 13. A compound X is formed by the reaction of a carboxylic acid C2H4O2 and an alcohol in presence of a few drops of H2SO4. The alcohol on oxidation with alkaline KMnO4 followed by acidification gives the same carboxylic acid as used in this reaction. Give the names and structures of (a) carboxylic acid, (b) alcohol and (c) the compound X. Also write the reaction.
Ans. (a) Carboxylic acid is ethanoic acid 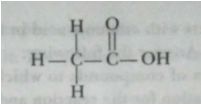
(b) Alcohol is ethanol 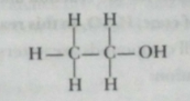
(c) X is ethyl ethanoate 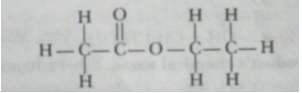
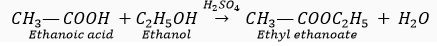
Que 14. An ester has the molecular formula C4H8O2. Write its structural formula. What happen when this ester is heated in the presence of sodium hydroxide solution? Write the balanced chemical equation for the reaction and name the products. What is a saponification reaction?
Ans. Structural formula: 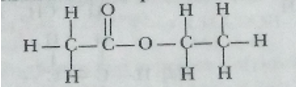
When this ester is heated in the presence of sodium hydroxide solution, it changes into an alcohol and a carboxylic acid.
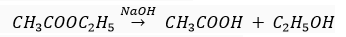
Products: Ethanol and ethanoic acid
Saponification: Reaction of an ester with an acid or base to give an alcohol and a carboxylic acid. This reaction is known as saponification because it is used in the preparation of soap.
Que 15. What is the role of metal or regents written on arrows in the given chemical reactions?
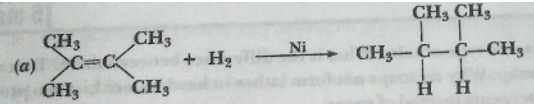
(b) 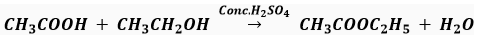
(c) 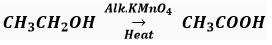
Ans. (a) Ni acts as a catalyst.
(b) Concentrated H2SO4 acts as a catalyst and a dehydrating agent.
(c) Alkaline KMnO4 acts as an oxidising agent.
Que 16. Write chemical equation of the reaction of ethanoic acid with the following: (a) Sodium; (b) Sodium hydroxide; (c) Ethanol.
Write the name of one main product of each reaction.
Ans. (a) 
(b) 
(c) 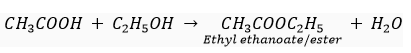
Que 17. When ethanol reacts with ethanoic acid in the presence of conc. H2SO4, a substance with fruity smell is produced. Answer the following:
(i) State the class of compounds to which the fruity smelling compounds belong. Write the chemical equation for the reaction and write the chemical name of the product formed.
(ii) State the role of conc. H2SO4 in this reaction.
Ans. (i) The fruity smell compounds are esters.
Chemical equation:

Product’s chemical name: Ethyl ethanoate.
(ii) Conc. H2SO4 acts as a dehydrating agent.
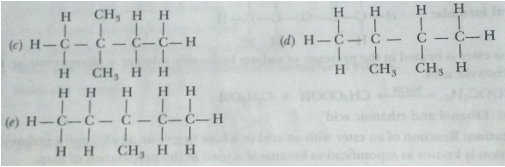
Que 18. Write the structural formulae of all the isomers of hexane.
Ans.