Short Answer Questions - II- 3 Marks
Q. 1. Write the cations and anions present (if any) in the following compounds:
(a) CH3COONa (b) NaCI
(c) H2 (d) NH4NO3
Ans. Anions Cations
(a) CH3COO- Na+
(b) Cl- Na+
(c) H2 - It is a covalent compound
(d)

Q. 2. Calculate the mass percentage of oxygen present in the following compounds and state the law of chemical combination associated. Given, H = 1, O = 16.
(i) Water (H2O) and (ii) Hydrogen peroxide (H2O2)
Ans. According to Law of multiple proportions
(i) H2O, % of O = 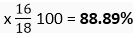
(ii) H202, % of O = 
Q.3. Classify each of the following on the basis of their atomicity.
(a) F2 (b) NO2 
(e) CO (f) H2O2 (g) P4O10 (h) O3
(i) HCI (j) CH4 (k) He (l) Ag
Ans. (a) 2 (b) 3 (c) 4 (d) 8
(e) 2 (f) 4 (g) 14 (h) 3
(i) 2 (j) 5
(k) 1 (Noble gases do not combine and exist as monoatomic gases)
(l) Polyatomic: It is difficuIt to talk about the atomicity of metals as any measurable quantity will contain millions of atoms bound by metallic bond.
Q.4. Calculate the molecular mass of the following:
(a) H2CO3 (b) C2H5OH (c) MgSO4
Ans. (a) Molecular mass of H2CO3 = 2 x 1 + 1 x 12 + 3 x 16
= 2 + 12 + 48
= 62 u
(b) Molecular mass of C2H5OH = 2 x 12 + 5 x 1 + 1 x 16 + 1
= 24 + 5 + 16 + 1
= 46 u
(c) Molecular mass of MgSO4 = 1 x 24 + 1 x 32 + 4 x 16
= 24 + 32 + 64
= 120 u
Q.5. What are ionic and molecular compounds? Give examples.
Ans. Atoms of different elements join together in definite proportions to form molecules of compounds. For example, water, ammonia, carbon dioxide. Compounds composed of metals and non-metals contain charged species. The charged species are known as ions. An ion is a charged particle and can be negatively or positively charged. A negatively charged ion is called an anion and the positively charged ion is called cation. For example, sodium chloride, calcium oxide.
Q.6. Give three significance of mole.
Ans. (a) One mole represents 6.022 × 1023 ntities of a substance.
(b) One mole of an element contains 6.022 × 1023 atoms of the element.
(c) One mole of a substance represents one gram formula mass of the substance.
Q.7. How many (a) molecules (b) hydrogen atoms (c) oxygen atoms are there in 0.5 mol of water?
Ans. (a) 1 mol of water contains 6.022 × 1023 molecules
∴ 0.5 mol of water contains 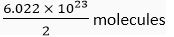
= 3.011 x 1023 molecules
(b) 1 molecule of water contains 2 atoms of hydrogen
1 mol of water contains 2 × 6.022 × 1023 atoms of hydrogen
∴ 0.5 mol of water contains 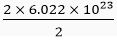
= 6.022 × 1023 atoms of hydrogen
(c) 1 molecule of water contains 1 atom of oxygen
1 mol of water contains 6.022 x 1023 atoms of oxygen
∴ 0.5 mol of water contains 
Q.8. Calculate the number of moles present in:
(i) 3.011 x 1023 number of oxygen atoms.
(ii) 60 g of calcium
[Given that atomic mass of Ca = 40 u, Avogadro No. = 6.022 × 1023]
Ans. (i) 1 mole of oxygen contains 6.022 x 1023 atoms
∴ 6.022 x 1023 atoms of oxygen = 1 mol
1 atom of oxygen = 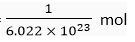
∴ 3.01 1 x 1023 atoms of oxygen = 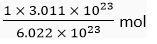
= 0.5 mol
(ii) Atomic mass of Ca = 40 u
40g of calcium = 1 mol
60g of calcium =  mol = 1.5 mol
mol = 1.5 mol
Q.9. Calculate the mass per cent of each element of sodium chloride in one mole of it.
Ans. Molecular mass of NaCl = (1 x 23 + 1 x 35.5) u = 58.5 u
Atomic mass of sodium = 23 u
Mass per cent of Na = 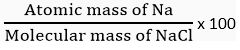

Mass % of Na = 39.32 %
Atomic mass of chlorine = 35.5 u
Mass % of Cl = 
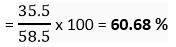
Q. 10. Calculate the number of particles in each of the following:
(a) 46 g of Na atom
(b) 8 g of O2 molecules
(c) 0.1 moles of carbon atom
Ans. (a) No. of moles of sodium = 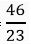 = 2 moles
= 2 moles
We know that one mole of sodium contains 6.022 x 1023 atoms.
∴ 2 moles of sodium contain = 2 x 6.022 x 1023 atoms
= 1.204 x 1024 atoms
(b) 1 mole of oxygen = 32 g
32 g of O2 contains 6.022 x 1023 molecules
∴ 8 g of O2 contains = 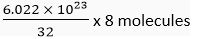
= 1.51 x 1023 molecules
(c) 1 mole of carbon atoms contains 6.022 × 1023 atoms
∴ 0.1 mole of carbon atoms contains = 6.022 x 1023 x 0.1 atoms
= 6.022 x 1022 atoms
Q. 11. Raunak took 5 moles of carbon atoms in a container and Krish also took 5 moles of sodium atoms in another container of same weight.
(a) Whose container is heavier?
(b) Whose container has more number of atoms?
Ans. (a) Mass of sodium atoms carried by Krish = (5 x 23) g = 115 g
Mass of carbon atoms carried by Raunak = (5 × 12) g = 60 g
Thus, Krish's container is heavier.
(b) Both the bags have same number of atoms as they have same number of moles of atoms.

