Short Answer Questions - II - 3 Marks
Q. 1. Substance 'A' has high compressibility and can be easily liquefied. It can take up the shape of any container. Predict the nature of the substance. Enlist four properties of this state of matter.
Ans. 'A' is a gas.
Properties of gases:
(i) They do not have fixed shape and fixed volume.
(ii) They have large interparticle space.
(iii) They have least forces of attraction between the molecules.
(iv) They are highly compressible.
Q. 2. Suggest an activity to show that the rate of diffusion of liquids decreases with increase in density of the liquid.
Ans.
- Take two beakers filled with water.
- Put a drop of blue ink slowly along the sides of the first beaker and honey in the same way in another beaker.
- Leave it undisturbed.
- We observe that honey diffuses slowly as compared to ink.
This experiment shows that lesser the density, faster the rate of diffusion.
Q. 3. Classify the following into osmosis/diffusion
(a) Swelling up of a raisin on keeping in water.
(b) Spreading of virus on sneezing.
(c) Earthworm dying on coming in contact with common salt.
(d) Shrinking of grapes kept in thick sugar syrup.
(e) Preserving pickles in salt.
(f) Spreading of smell of cake being baked throughout the house.
(g) Aquatic animals using oxygen dissolved in water during respiration.
Ans. (a) Osmosis
(b) Diffusion
(c) Osmosis
(d) Osmosis
(e) Osmosis
(f) Diffusion
(g) Diffusion
Q. 4. Explain what happens to the molecular motion and energy of 1 kg of water at 273 K when it is changed into ice at same temperature. How is the latent heat of fusion related to the energy exchange that takes place during this change of state?
Ans."
- Molecular motion decreases as water gets converted into ice.
- Latent heat of solidification is given off.
- Latent heat of solidification is equal to latent heat of fusion.
Q. 5. Design an experiment to show that ammonium chloride
undergoes sublimation.
Ans.
- Take crystals of ammonium chloride in a china dish.
- Put the China dish on a tripod stand with wire gauze.
Put an inverted funnel on the china dish and insert a- cotton plug in the stem of the funnel.
- Heat the china dish on a low flame.
In the inside of the funnel white deposits of ammonium
chloride is seen which directly converts into gaseous state- and then solidifies.

Q. 6. Explain interconversion of three states of matter with the help of flow chart. Name the process of each interconversion.
Ans.
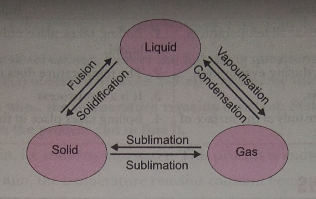
Q. 7. A student heats a beaker containing ice and water. He measures the temperature of the content of the beaker as a function of time. Which of the following (shown in figure given below) would correctly represent the result? Justify your choice.

Ans. Since ice and water are in equilibrium, the temperature would be zero. When we heat the mixture, energy supplied is utilised in melting the ice and the temperature does not change till all the ice melts because of latent heat of fusion. On further heating, the temperature of the water would increase. Therefore, the correct option is (d).
Q. 8. Explain how the rate of evaporation of a liquid is affected with:
(i) Increase in temperature of the liquid.
(ii) Decrease in exposed surface area.
(iii) Increase in moisture in the surrounding air.
(iv) Increase in wind speed.
Ans.
(i) Rate of evaporation increases with rise in temperature.
(ii) Evaporation is less when exposed surface area decreases.
(iii) Less evaporation if moisture content is high in the air.
(iv) Rate of evaporation increases if wind speed increases.
Q.9. You want to wear your favourite shirt to a party, but the problem is that it is still wet after a wash. What steps would you take to dry it faster?
Ans. Conditions that can increase the rate of evaporation of water are:
(a) An increase in the surface area by spreading the shirt.
(b) An increase in the temperature by putting the shirt under the sun.
(c) Increase in the wind speed by spreading it under the fan.
Q. 10. How does evaporation differ from boiling?
Ans.
|
Evaporation |
Boiling |
|
1. Evaporation takes place at all temperatures. 2. Temperature changes during evaporation. 3. It is a very slow process. 4. Evaporation takes place only at the surface of the liquid. |
1. Boiling takes place only at the boiling point of the liquid. 2. The temperature does not change during boiling. 3. It is a fast process. 4. Boiling takes place in the entire body of the liquid. |

