Short Answer Questions - I - 2 Marks
Que 1. What is an olfactory indicator? Name two such indicators.
Ans. Those substances whose smell (or odour) changes in acidic or basic solution are called olfactory indicators onion and vanilla extract are olfactory indicators.
Que 2. List in tabular form differences between an acid and a base based on their chemical properties.
Ans.
|
Acids |
Bases |
|
1. H+ ions are released in aqueous solution.
|
1. OH- ions are released in aqueous solution.
|
Que 3. What happens when a cold and concentrated solution of sodium chloride reacts with ammonia and carbon dioxide? Write the equation of the reaction which takes place.
Ans. When a cold and concentrated solution of sodium chloride reacts with ammonia and carbon dioxide, sodium hydrogencarbonate (baking soda) and ammonium chloride is formed.
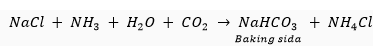
Que 4. Name the gas evolved when dilute HCl reacts with sodium hydrogen carbonate. How is it recognised?
Ans. CO2 gas is evolved. The reaction is as follows:
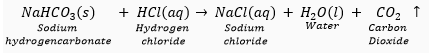
When the produced gas is passed through lime water, lime water turns milky which confirms that the evolved gas is CO2.
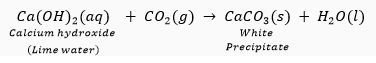
Que 5. The pH of soil A is 7.5 while that of soil B is 4.5. Which of the two soils should be treated with powdered chalk to adjust its pH and why?
Ans. Soil B having pH 4.5 should be treated with powdered chalk to adjust its pH. Soil B is too acidic. Its acidity can be reduced by adding powdered chalk (CaCO3), which is a base.
Que 6. What is 'baking powder'? How does it make the cake soft and spongy?
Ans. Baking powder is a mixture of baking soda (NaHCO3) and an edible acid like tartaric acid. Baking powder on heating produces carbon dioxide gas which causes bread or cake to rise making it soft and spongy.
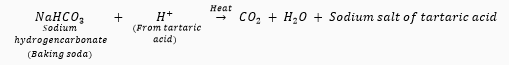
Que 7. The conditions preferred by some plants are shown in the table below:
|
Plant |
Apple |
Potato |
Black currant |
Mint |
Onion |
Strawberry |
Lettuce |
|
pH |
5.0-6.5 |
4.5-6.0 |
6.0-8.0 |
7.0-8.0 |
6.0-7.0 |
5.0-7.0 |
6.0-7.0 |
(a) Which plants grow well over the largest range pf pH values?
(b) Which plant can grow in the most acidic soil?
(c) Which plant can grow in the basic soil only?
(d) What is the pH range for onion to grow well?
Ans. (a) Black currant and strawberry
(b) Potato (4.5-6.0)
(c) Mint (7.0-8.0)
(d) 6.0-7.0
Que 8. (i) Name the products formed when sodium hydrogencarbonate is heated.
(ii) Write the chemical equation for the reaction involved in it.
Ans. (i) On heating sodium hydrogencarbonate (NaHCO3), it decomposes to form sodium carbonate (Na2CO3), water (H2O) and carbon dioxide (CO2).
(ii) 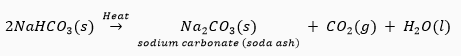
Que 9. Name the acid present in an ant sting and give its chemical formula. Also give the common method to get relief from the discomfort caused by the ant sting.
Ans. The acid present in an ant sting is methanoic acid (formic acid). The chemical formula is HCOOH. To get relief, one should apply any available basic salt, e.g., baking soda (NaHCO3) on it.
Que 10. What happens when nitric acid is added to an eggshell?
Ans. Eggshell contain calcium carbonate. When nitric acid is added to it, carbon dioxide gas is evolved. The reaction can be gives as
CaCO3 + 2HNO3

