Short Answer Questions - I - 2 Marks
Q. 1. How do you know that nucleus is very small as compared to the size of atom?
Ans. Rutherford observed that when α-particles were bombarded on a very thin foil they bounced back. But the number of a-particles bouncing back got doubled when he doubled the thickness of gold foil. Then he concluded that the area of nucleus is very small in comparison to the total area of the atom.
Q. 2. Write two characteristics of the canal rays.
Ans. (i) The canal rays are deflected by the magnetic fields in a direction opposite to that of the cathode rays.
(ii) They consist of positively charged particles.
Q.3. Write the electronic configuration of a positively charged sodium ion (Na+). Atomic number of sodium is 11.
Ans. Number of electrons in Na atom = Atomic number = 11
Number of electrons in Na+ ion = 1 1 - 1 = 10
Electronic configuration of Na+ ion: 2, 8
Q.4. The electronic configuration of phosphorus atom is 2, 8, 5. Give the electronic configuration of P3- ion.
Ans. Electronic configuration of P = 2, 8, 5
P atom gains 3e- to form P3-
∴ P3- has configuration = 2, 8, (5 + 3) = 2, 8, 8
Q.5. The atomic number of Al and Cl are 13 and 17, respectively. What will be the number of electrons in Al3+ and Cl-?
Ans. Atomic number of Al = Number of electrons = 13
Number of electrons in Al3+ = 13 - 3 = 10
Atomic number of chlorine = Number of electrons = 1 7
Number of electrons in CI- = 17 + 1 = 18
Q.6. Write down the electron distribution of chlorine atom. How many electrons are there in the L shell? (Atomic number of chlorine is 17).
Ans. The electronic distribution of Cl is 2, 8, 7. The L shell has eight electrons.
Q. 7. Define valence electrons. Which electrons of an atom are invoved in the chemical bond formation with other atoms?
Ans. The electrons present in the outermost shell of an atom or ion are known as valence electrons. In a chemical bond formation, only valence electrons of an atom take part.
Q.8. Why do helium, neon and argon have a zero valency?
Ans. Helium has two electrons in its energy shell, while argon and neon have 8 electrons in their valence shells. As these have maximum number of electrons in their valence shells, they do not have any tendency to combine with other elements. Hence, they have a valency equal to zero.
Q.9. Helium atom has 2 electrons in its valence shell but its valency is not 2. Explain.
Ans. Helium atom has 2 electrons in its valence shell and its duplet is complete. Hence, the valency is zero.
Q. 10. Find out the valency of the atoms represented by the Figs. (a) and (b)
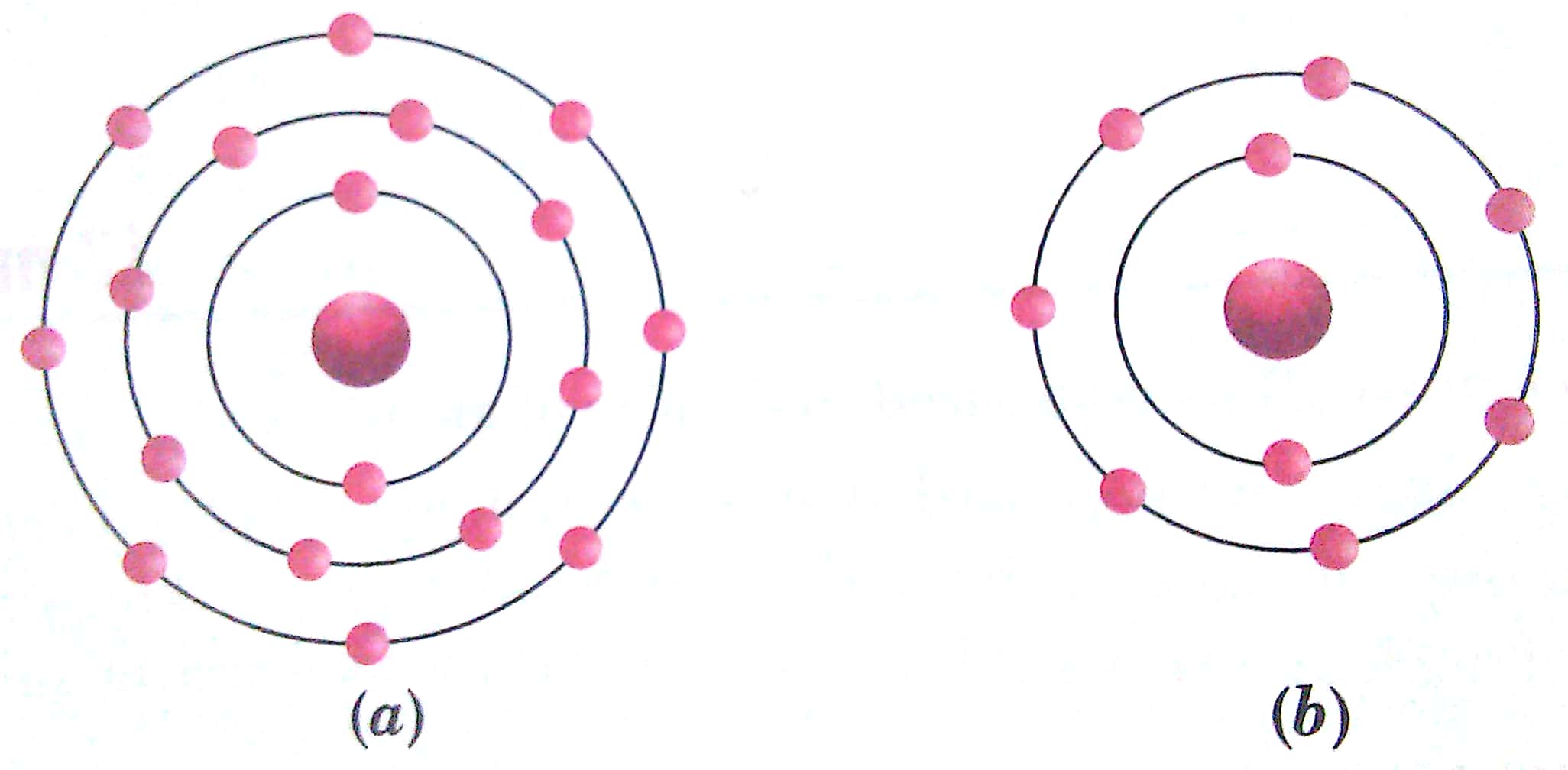
Ans. (a) 0 (b) 1
Q.11. Identify the Na+ ion from the following figures. What is the valency of sodium atom? Give reason.

Ans. Figure number (ii) is correct because sodium ion (Na+) is formed when one electron is lost.
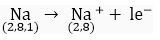
The valency of sodium atom is one because stable (octet) electronic configuration is obtained after loss of one electron.
Q. 12. Calculate the number of neutrons present in the nucleus of an element X which is represented as 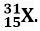
Ans. Mass number = No. of protons + No. of neutrons = 31
∴ Number of neutrons = 31 – Number of protons
= 31 – 15 = 16
Q. 13. Why do isotopes show similar chemical properties?
Ans. Isotopes have same atomic numbers and thus same number of electrons. Therefore, they have the same electronic configuration which provides them similar chemical properties.
Q. 14. An element 'X' has a valency 3(+):
(a) Write the formula of its phosphide.
(b) Write the formula of its carbonate.
Ans. (a) XP (b) X2 (CO3)3
Q. 15. An element "Z' forms the following compound when it reacts with hydrogen, chlorine, oxygen and phosphorous.
ZH3, ZCl3, Z203, and ZP
(a) What is the valency of element ‘Z'?
(b) Element 'Z' is metal or non-metal?
Ans. (a) The valency of ‘Z' is 3.
(b) Element ‘Z’ is a metal because it is electropositive and is reacting with non-metals.

