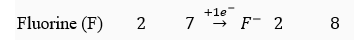Short Answer Questions - I - 2 Marks
Que 1. The three elements A, B and C with similar properties have atomic masses X, Y and Z respectively. The mass of Y is approximately equal to the average mass of X and Z. What is such an arrangement of element called as? Give one example of such a set of elements.
Ans. The arrangement of these elements is known as Döbereiner triad. Example: lithium, sodium and potassium.
Que 2. Elements have been arranged in the following sequence on the basis of their increasing atomic masses
F, Na, Mg, Al, si, P, S, Cl, Ar, K
(a) Pick two sets of elements which have similar properties.
(b) The given sequence represents which law of classification of elements?
Ans. (a) (i) F and CI
(ii) Na and K
(b) Newlands' Law of Octaves
Que 3. Why did Mendeléev leave some gaps in his Periodic Table?
Ans. Mendeléev left some gaps in his Periodic Table for the elements yet to be discovered. He even predicted the properties of these element by studying the properties of the neighbouring elements.
Que 4. State Periodic Law on which the Modern Periodic Table is based.
Ans. Modern periodic Law based on atomic number can be stated as "properties of the elements are periodic function of their atomic number." That is, in the Modern Periodic Table, the elements are arranged in the order of their increasing atomic numbers.
Que 5. What do you understand by the term periodicity? Does the periodicity in properties is a function of valence electrons? Illustrate.
Ans. When the elements are arranged in order of increasing atomic numbers, elements with similar chemical properties are repeated at definite intervals. This is known as periodicity. Yes, this periodicity is due to the periodicity in the number of electrons in the outermost shell of the atoms of the elements. If elements having the same number of valence electrons are grouped together, the elements falling within each group are similar in chemical properties.
Que 6. What does each group in the Periodic Table signify?
Ans. Each group in the Periodic Table signifies identical outer shell electronic configuration for the elements constituting that group. For example, each element of group I has one electron in its outermost shell.
Que 7. Why do elements in any given group have similar properties?
Ans. The chemical properties of an atom are largely determined by its valence electrons. In a given group, the number of valence electrons are same, hence they have similar properties
Que 8. Why do group 1 elements form unipositive ions?
Ans. Group 1 elements contain 1 electron in their outermost shells. These elements lose this electron easily to attain the 8 electrons in their outermost shell. Hence, they form unipositive ion
For example,
K L M K L
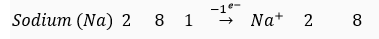
Que 9. Why are the elements of group 18 called zero valent?
Ans. Group 18 elements have their outermost shells completely filled and the atoms of these elements have no tendency to gain or lose electrons. Thus, the elements of this group are zero valent and almost unreactive.
Que 10. Why group 17 elements form uninegative anions?
Ans. Group 17 elements have 7 valence electrons, one electron less than the maximum number of electrons that can be accommodated in the outermost shell. Therefore, it is easier for these elements to gain an electron and form uninegative anions, so as to attain noble gas configuration
For example, K L K L