Short Answer Questions - I - 2 Marks
Q. 1. Give an example to show law of conservation of mass applies to physical changes also.
Ans. Law of conservation of mass states that mass can neither be created nor destroyed in a chemical reaction. However, this law applies to physical changes also. For example, when ice melts into water, the mass of ice equals to the mass of water, i.e., the mass is conserved. This verifies the law of conservation of mass.
Q. 2. Which of the following symbols of elements are incorrect? Give their correct symbols.
(a) Cobalt CO (b) Carbon c
(c) Aluminium AL (d) Helium He
(e) Sodium So
Ans.
(a) Incorrect, the correct symbol of cobalt is Co.
(b) Incorrect, the correct symbol of carbon is C.
(c) Incorrect, the correct symbol of aluminium is Al.
(d) Correct (He)
(e) Incorrect, the correct symbol of sodium is Na.
Q.3. which of the following are tri-atomic and tetra-atomic molecules?
CH3CI, CaCl2, NH3, PCl3, P205, H2O, C2H5OH
Ans. (i) Tri-atomic molecules are CaCl2, H2O.
(ii) Tetra-atomic molecules are NH3, PC13.
Q. 4. Differentiate between the actual mass of a molecule and gram molecular mass.
Ans. Actual mass of a molecule is obtained by dividing the molar mass by Avogadro's number whereas gram molecular mass represents the molecular mass expressed in grams, i.e., it is the mass of 1 mole of molecules, i.e., Avogadro's number of molecules.
Q. 5. Calculate the formula mass of sodium carbonate (Na2CO3.10H20).
Ans. Formula mass of sodium carbonate
= (2 x atomic mass of Na) + (1 x atomic mass of C) + (3 x atomic mass of O)
+ 10 [(2 x atomic mass of H) + (1 x atomic mass of O)]
= 2 x 23 + 1 x 12 + 3 x 16 + 10 [(2 x 1) + (1 x 16)]
= 46 + 12 + 48 + 180 = 286 u
Q. 6. Calculate the mass of one atom of hydrogen atom.
Ans. 1 mole of hydrogen atom = 1g
or 6.022 × 1023 atoms of hydrogen weigh = 1 g
Mass of one atom 
= 1.66058 × 10-24 g
Q. 7. How many moles are present in 4 g of sodium hydroxide?
Ans. Gram molar mass of NaOH = 23 + 16 + 1 = 40 g
40 g of NaOH = 1 mol
∴ 1 g of NaOH = 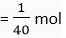
∴ 4 g of NaOH 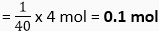
Q.8. A sample of ammonia weighs 3.00 g. What mass of sulphur trioxide contains the same number of molecules as are in 3.00 g ammonia?
Ans. Number of moles of ammonia in 3.00 g 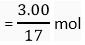
= 0.1764 mol
Molecular mass of SO3 = 1 × 32 u + 3 × 16 u = 80 u
l mole of SO3 weighs 80 g
∴ 0.1764 moles weigh = 80 x 0.1764 g
= 14.11 g
Q. 9. Carbon dioxide produced by action of dilute hydrochloric acid on potassium hydrogen carbonate is moist whereas that produced by heating potassium hydrogen carbonate is dry. What would be the difference in the composition of carbon dioxide in the two cases? State the associated law.
Ans. The composition of CO2 in both the cases would be same,ie., the carbon and oxygen will combine in the same ratio 1 : 2.
The law associated is law of constant proportion.
Q. 10. How many atoms would be present in a black dot marked on the paper with graphite pencil as a full stop at the end of a sentence.
[Given mass of a dot = 10-18 g]
Ans. I mole of carbon atoms weigh = 12 g
Also, 1 mole of carbon atoms = 6.022 x 1023 atoms
Thus, 12 g of carbon atoms has 6.022 x 1023 atoms.
∴ 10-18 g of carbon will have 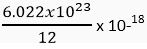
= 5.02 × 104 carbon atoms.
Q. 11. Does the solubility of a substance change with temperature? Explain with the help of an example.
Ans. Yes, it is a temperature dependent property. The solubility generally, increases with increase in temperature. For example, you can dissolve more sugar in hot water than in cold water.

