Short Answer Questions - I - 2 Marks
Q. 1. When a crystal of potassium permanganate is placed in a beaker containing water, its purple colour spreads throughout the water. What do you conclude from this observation about the nature of potassium permanganate and water?
Ans. When we place few crystals of potassium permanganate in a beaker containing water, we get two distinct layers-colourless water at the top and pink colour at the bottom. After few minutes, pink colour spreads and whole solution turns pink due to diffusion. Since potassium permanganate is a solid substance, it does not possess so much space. Water molecules due to liquid state, collide with solid particles and intermix due to sufficient space between molecules.
Q. 2. Why do solids have a regular geometrical shape?
Ans. In solids, the particles have highly ordered arrangement because the intermolecular forces between the particles are very strong. Therefore, solids have a regular geometrical shape.
Q. 3. Why are gases compressible but not liquids?
Ans. Cases are compressible because the intermolecular space is very large in gases, whereas liquids are not compressible because in liquids, the intermolecular space is less.
Q. 4. Can a rubber band change its shape on stretching? Is it a solid?
Ans. Yes, a rubber band changes shape under force and regains the same shape when the removed. It breaks on applying excessive force. Yes, it is a solid.
Q.5. Why steam at 100°C is better for heating purposes than water at 100°C?
Ans. Steam at 100°C is better for heating purposes than water at 100°C because the energy of 1 steam at 100°C is 22.6 × 100 Joule which is more than that of 1 kg of water at the temperature.
Q. 6. Give two ways in which melting points and boiling points can be useful.
Ans. (a) To check whether the substance is pure or not.
(b) To identify and characterise the substance
Q. 7. Alka was making tea in a kettle. Suddenly she felt intense heat from the puff of steam gushing out of the spout of the kettle. She wondered whether the temperature of the steam was higher than that of the water boiling in the kettle. Comment.
Ans. The temperature of both boiling water and steam is 100° C, but steam has more energy because of latent heat of vaporisation.
Q. 8. Why does the temperature of a substance remain constant during its melting point or boiling point?
Ans. The temperature of a substance remains constant at its melting and boiling point until all the substance melts or boils because, the heat supplied is continuously used up in changing the state of the substance by overcoming the forces of attraction between the particles. This heat energy absorbed without showing any rise in temperature is given the name latent heat of fusion/latent heart of Vaporisation.
Q.9. What do you understand by the term ‘latent heat of fusion'? How much is the latent heat of fusion of ice?
Ans. The amount of heat that is required to change 1 kg of solid into liquid at atmospheric pressure without any change in temperature at its melting point, is known as latent heat of fusion. The latent heat of fusion of ice in SI unit is 3.35
 105 J/kg.
105 J/kg.
Q. 10 which gas is called dry ice? Why?
Ans. Solid Co2 Is known as dry ice. This is because it directly gets converted into gaseous state without passing through liquid state on decreasing the pressure to 1 atmosphere.
Q. 11 A glass tumbler containing hot water is kept in the freezer compartment of a refrigerator ( temperature <00C) . If you could measure the temperature of the content of the tumbler, which of the following graphs would correctly represent the change in its temperature as a function of time?

Ans. (a). The water will cool initially till it reaches 00C, the freezing point. At this stage, the temperature will remain constant till all the water will freeze. After this, temperature would fall again.
Q.12. Why do the doctors advise to put strips of wet cloth on the forehead of a person having high fever?
Ans. When a person has fever, his body temperature becomes more than the normal body temperature. If we put strips of wet cloth on the forehead of a person suffering from high fever, the water evaporates taking heat from the body. Thus, moist strips will lower his body temperature.
Q.13. Look at the following figures and suggest in which of the glass containers, i.e., A, B, C or D, the rate of evaporation will be the highest? Explain.
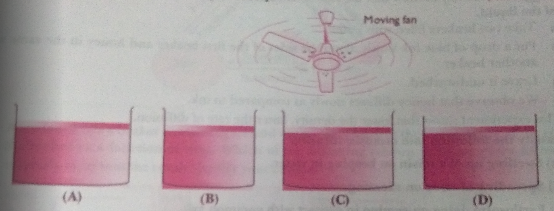
Ans. (C). The rate of evaporation increases with an increase in surface area because evaporation is a surface phenomenon. Also, with the increase in air speed, the particles of water vapour will move away with air, which will increase the rate of evaporation.
Q. 14. Why do wet clothes dry quickly in the sun than in the shade?
Ans. The temperature in the sunny area is higher than in the shade and evaporation takes place at a faster rate at high temperature. Hence, wet clothes dry quickly in the sun.
Q. 15. Why do trees acquire more leaves during summer?
Ans. During summer the temperature is generally very high. In order to keep cool, a tree must transpire (transpiration is a phenomenon of evaporation of water from the leaves) more to keep itself cool. More transpiration requires more leaves. Hence, a tree acquires more leaves during summer.
Q. 16. Why do we feel comfortable under a fan when we are perspiring?
Ans. The sweat is readily evaporated from the body by the air from the fan. As a result, we feel comfortable under a fan.
Q. 17. Why do people sprinkle water on the roof after a hot sunny day?
Ans. Water sprinkled on the roof evaporates by taking the large latent heat of vaporisation from the ground. This makes the place cool and comfortable.
Q. 18. It is a hot summer day, Priyanshi and Ali are wearing cotton and nylon clothes respectively. Who do you think would be more comfortable and why?
Ans. Priyanshi would be more comfortable because cotton is a good absorber than nylon, It absorbs sweat from the body and provides large surface area for evaporation which causes cooling effect. As a result, body feels cool and comfortable.

