Short Answer Question - II - 3 Marks
Q. 1. List any three distinguishing features between the models of an atom proposed by J.J. Thomson and Ernest Rutherford.
Ans.
|
J. J. Thomson Model of Atom |
Rutherford's Model |
|
1. Positive charge forms a kernel. 2. Electrons present throughout the atom. 3. No space is empty. |
1. Nucleus (positive charge) is in the centre. 2. Electrons revolve in orbits. 3. Most of the space is empty. |
Q.2. In the gold foil experiment of Geiger and Marsden, that paved the way for Rutherford's model of an atom, ~1.00% of the a-particles were found to deflect at angles > 50°. If one mole of a-particles were bombarded on the gold foil, compute the number of a-particles that would deflect at angles less than 50°.
Ans. % of a-particles deflected more than 500 = 1% of a-particles.
% of a-particles deflected less than 50° = 100 - l = 99%
Number of particles that deflected at an angle less than 500


= 5.96 x 1023
Q.3. Predict the valency of the following elements
(i) A (Atomic number 5) (ii) B (Atomic number 12)
(iii) C (Atomic number 14) (iv) D (Atomic number 17)
Ans. (i) Valency of element ‘A' = 8 - 5 = 3
(ii) Valency of element ‘B' = 12 - 10 = 2
(iii) Valency of element ‘C' = 14 - 10 = 4
(iv) Valency of element ‘D' = 18 - 17 = 1
Q. 4. An element 'X' contains 6 electrons in 'M' shell as valence electrons:
(a) What is the atomic number of ‘X’?
(b) Identify whether ‘X' is a metal or non-metal.
Ans. (a) If ‘X’ contains 6 electrons in 'M' shell as valence electrons, then the electronic configuration of ‘X’ is K = 2, L = 8, M = 6
∴ Atomic number = 16
(b) ‘X' is a non-metal.
Q 6. Complete the table on the basis of information available in the symbols given below

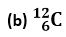

|
Element |
np |
nn |
|
|
|
|
|
|
|
|
|
|
|
|
Ans.
|
Element |
np |
nn |
|
Cl |
17 |
18 |
|
C |
6 |
6 |
|
Br |
35 |
46 |
Q.7. In the atom of an element 'Z', 5 electrons are present in the outermost shell . It requires noble gas configuration by accepting requisite number of electrons, then what would be the charge on the ion so formed? Write the formula of the compound which will be formed when 'Z' reacts with Na atom.
Ans. Number of electrons in the outermost shell = 5
Number of electrons required to make noble gas configuration = 8 – 5 = 3
The charge on the ion so formed = Z + 3e-
= Z3-
The valency of Z = 3
Chemical formula of the compound: 
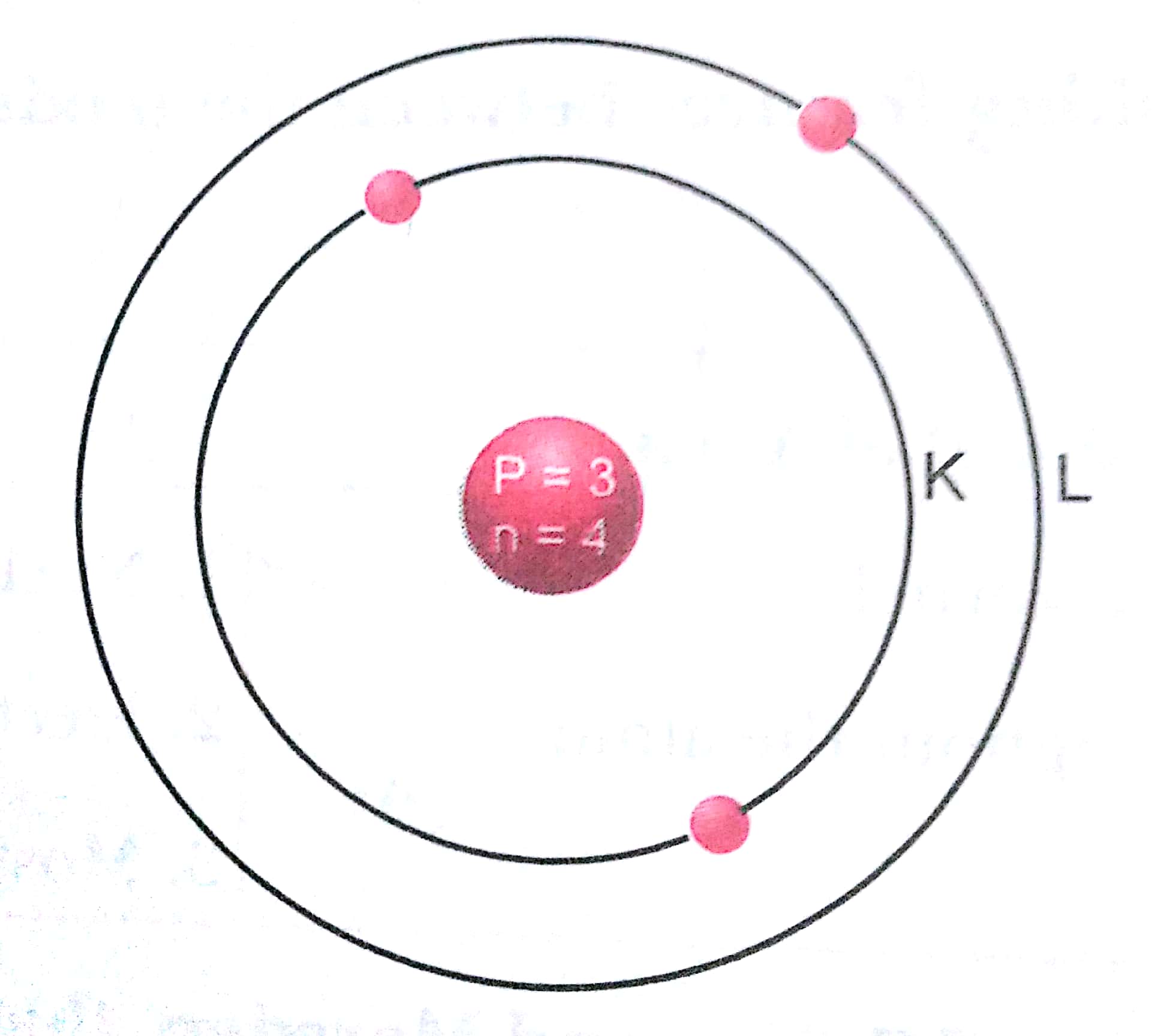
Q. 8. Rn is an isotope of noble gas, radon. How many protons, neutrons and electrons are there in one atom of this radon isotope?
Ans. Atomic number of radon = 86
The number of protons = 86
The number of electrons = Number of protons
= 86
Number of neutrons = Atomic mass - Atomic number
= 222 - 86
= 136
Q. 9. What information do you get from the figures about the atomic number, mass number and valency of atoms X, Y and Z? Give your answer in a tabular form.
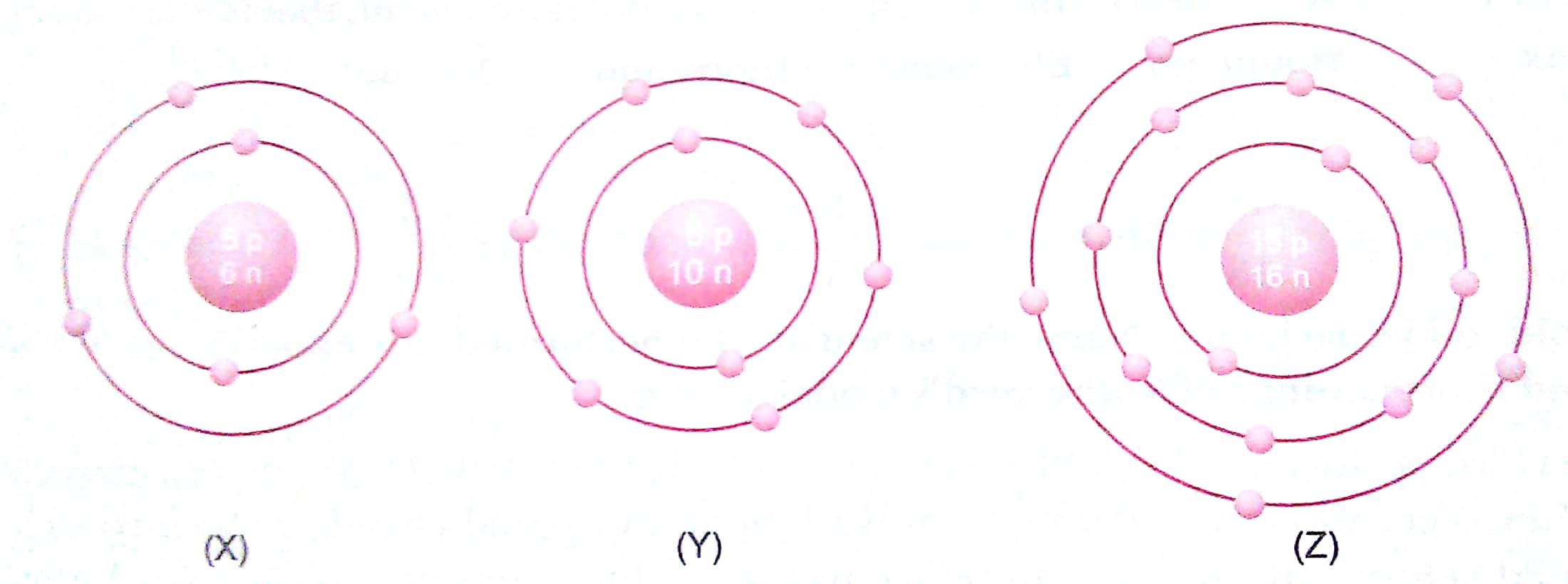
Ans.
|
|
Atomic No. |
Mass No. |
Valency |
|
X |
5 |
11 |
3 |
|
Y |
8 |
18 |
2 |
|
Z |
15 |
31 |
3, 5 |
Q. 10. Write the molecular formulae for the following compounds:
(a) Copper (II) bromide (b) Aluminium (III) nitrate
(c) Calcium (II) phosphate (d) Iron (III) sulphide
(e) Mercury (II) chloride (f) Magnesium (II) acetate
Ans. (a) CuBr2 (b) Al(NO3)3
(c) Ca3(PO4)2 (d) Fe2S3
(e) HgCl2 (f) Mg(CH3COO)2
Q. 11. Write the molecular formulae of all the compounds that can be formed by the combination of following ions
![]()
Ans. CuCl2; CuSO4; Cu3 (PO4)2
NaCl; Na2SO4; Na3PO4
FeCl3; Fe2(SO4)3; FePO4
Q. 12. Write the formula of the compounds formed by the following ions.
(a) Mg2+ and s2- (b) Cu2+ and OH-
Name the compounds formed in each case.
Ans. (a) lons Mg2+ S2-
Valencies 2 2
Compound: Mg2S2 or MgS; Magnesium sulphate
(b) Ions Cu2+ OH-
Valencies 2 1
Compound: Cu (OH) 2; Copper (II) hydroxide.

