Periodic Classification of Elements
Que 1. Using the part of the periodic Table given below, answer the questions that follow:
|
Groups |
1 |
2 |
13 |
14 |
15 |
16 |
17 |
18 |
|
1 |
H |
|
|
|
|
|
|
He |
|
2 |
Li |
Be |
B |
C |
N |
O |
F |
Ne |
|
3 |
Na |
Mg |
Al |
Si |
P |
S |
Cl |
Ar |
|
4 |
K |
Ca |
|
|
|
|
|
|
(i) Na has physical and chemical properties similar to which element (s) and why?
(ii) Write the electronic configure of N and P. Which one of these will be more electronegative and why?
(iii) State a chemical property common to fluorine and chlorine.
Ans. (i) Lithium and potassium, due to same number of valence electrons.
(ii) N  2, 5
2, 5
P
N is more electronegative element as electronegative decreases on moving down the group.
(iii) Both fluorine and chlorine form their hydracids on reacting with hydrogen.
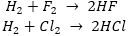
Que 2. Is it possible to have an element with atomic number 2.5?
Ans. No, because the atomic numbers of elements are always in whole numbers.
Que 3. An elements ‘X’ has mass number 35 and number of neutrons 18. Write number and electronic configure of ‘X’. Also write group number, period number and valency of ‘X’.
Ans. Atomic number of X = mass number of X – Number of neutrons
= 35 – 18 = 17
Therefore, electrons configure of X = 2, 8, 7
Group number = 17
Period number = 3
Valency = 8 – 7 = 1
Que 4. Three elements A, B and C have 3, 4 and 2 electrons respectively in their outermost shell. Give the group number to which they belong in the modern periodic Table. Also, give their valencies.
Ans.
|
Element |
Group Number |
Valency |
|
A |
13 |
3 |
Que 5. Compare the radii of two species X and Y. Give reasons for your answer.
(a) X has 12 protons and 12 electrons
(b) Y has 12 protons and 10 electrons
Ans. Radii of Y is less than X because Y is cation of X.
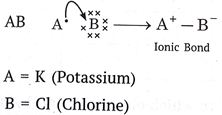
Que 6. Write the formula of the product formed when the element A (atomic numbers 19) combines with the element B (atomic number 17). Draw its electronic dot structure.
What is the nature of the bond formed?
Ans.
Que 7. An element X (atomic number 17) reacts with an element Y (atomic number 20) to form a divalent halide.
(a) Where in the Periodic Table are elements X and Y placed?
(b) Classify X and Y as metal (s), non-metal (s) or metalloid (s).
(c) What will be the nature of oxide of element Y? Identify the nature of bonding in the compound formed.
(d) Draw the electron dot structure of the divalent halide.
Ans. (a) X belongs to Group 17 and 3rd period
Y belongs to Group 2 and 4th period
(b) X-Non-metal and Y-Metal
(c) Basic oxide; Ionic bonding
(d)

Que 8. (i) Electropositive nature of the element(s) increases down the group and decreases across the period
(ii) Electronegativity of the element decreases down the group and increases across the period
(iii) Atomic size increases down the group and decreases across a period (left to right)
(iv) Metallic character increases down the group and decreases across a period.
On the basis of the above trends of the Periodic Table, answer the following about the elements with atomic numbers 3 to 9.
(a) Name the most electropositive element among them
(b) Name the most electronegative element.
(c) Name the element with smallest atomic size.
(d) Name the element which is a metal loid.
(e) Name the element which shows maximum valency
Ans. (a) Lithium (b) Fluorine
(c) Fluorine (d) Boron
(e) Carbon
Que 9. In the following table, the positions of six elements A, B, C, D, E and F are given as they are in the Modern Periodic Table:
|
Group |
1 |
2 |
3 – 12 |
13 |
14 |
15 |
16 |
17 |
18 |
|
2 |
A |
|
|
B |
|
C |
|
|
D |
|
3 |
|
|
|
|
E |
|
|
|
F |
On the basis of the above table, answer the following questions:
(i) Name the element which forms only covalent compounds.
(ii) Name the element which is a metal with valency three.
(iii) Name the element which is a non-metal with valency three.
(iv) Out of B and C, whose atomic radius is bigger and why?
(v) Write the common name for the family to which the elements D and F belong.
Ans. (i) E
(ii) B
(iii) C
(iv) B, because atomic radius decreases from left to right due to increase in the nuclear charge.
(v) Noble gases
Que 10. Two elements 'A' and 'B' belong to the 3rd period of Modern periodic table and are in group 2 and 13 respectively. Compare their following characteristics in tabular form:
(a) Number of electrons in their atoms
(b) Size of their atoms
(c) Their tendencies to lose electrons
(d) The formula of their oxides
(e) Their metallic character
(f) The formula of their chlorides
Ans.
|
|
Characteristics |
A |
B |
|
(a) |
Number of electrons in their atoms |
4 or 12 or 20 |
5 or 13 or 21 |
|
(b) |
Size of their atoms |
Bigger |
Smaller |
|
(c) |
Their tendencies to lose electrons |
More |
Less |
|
(d) |
The formula of their oxides |
AO |
B2O3 |
|
(e) |
Their metallic character |
More metallic |
Less metallic |
|
(f) |
The formula of their chlorides |
ACl2 |
BCl3 |



