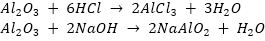Metals & Non - Metals
Que 1. There are 3 unknown metals-A, B and C. C displaces B from its oxide while with oxide of A, there is no reaction. Give the reactivity order of A, B and C.
Ans. C displaces B from its oxide, therefore, C is more reactive than B.
There is no reaction when C is treated with oxide of A or C does not displace A from its oxide. So, A is more reactive than C. Thus, the reactivity order is B < C < A.
Que 2. A copper coin is kept immersed in a solution of silver nitrate for some time. What will happen to the coin and the colour of solution?
Ans. Copper is placed above the silver in the reactivity series which indicated that copper is more reactive than silver. When a copper coin or strip is kept immersed in a solution of silver nitrate (AgNO3), silver from its solution will deposit on copper coin.
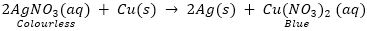
Copper slowly displaces silver from the AgNO3 solution and the colour of solution changes from colourless to blue due to formation of copper nitrate [Cu(NO3)2. The copper con will disappear and silver will precipitate out.
Que 3. An element A reacts with water to from a compound B which is used in white-washing. The compound B on heating forms an oxide C which on treatment with water gives back B. Identify A, B and C and give the reactions involved.
Ans. A is calcium, it reacts with water to form calcium hydroxide which is used in white washing. So, B is Ca (OH) 2.
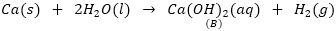
On heating compound B, i.e., calcium hydroxide, it forms calcium oxide, i.e., C.
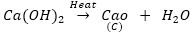
Que 4. ‘M’ is an element which is out of Cu, Fe, Al, Na. It shows the following properties:
(i) One of its ore is rich in M2O3.
(ii) M2O3. Is not affected by water.
(iii) It corrodes easily.
(iv) It forms two chlorides MCl2 and MCl3. Identify ‘M’.
Ans. As the metal ‘M’ forms oxide m2O3 it is trivalent. Out of the metals listed, only Fe and Al are trivalent.
M2O3 is not affected by water, so ‘M’ can be out of Fe or Al.
Fe and Al both corrode easily.
Out of Al and Fe, only Fe can form divalent chloride, so the element ‘M’ is Fe.
Que 5. Carbon can reduce copper oxide to copper but not CaO to Ca. Why?
Ans. C is a strong reducing agent and can reduce CuO as follows:
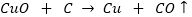
Ca is much more reactive than Cu and has greater affinity for oxygen than C has. So, carbon cannot reduce CaO to Ca.
Que 6. A metal A, which is used in thermit process, when heated with oxygen gives an oxide B, which is amphoteric in nature. Identify A and B. Write down the reactions of oxide B with HCl and NaOH.
Ans. A is aluminium (Al). It reacts with oxygen to form aluminium oxide, Al2O3.
4Al(s) + 3O2 (g)  2Al2O3(s)
2Al2O3(s)
So, B is Al2O3.