Matter in Our Surroundings
Que 1. The diagram below shows burning of an oil lamp.
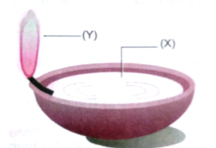
Draw the arrangement of particles of position ‘X’ and ‘Y’ when the lamp is burning.
Ans.
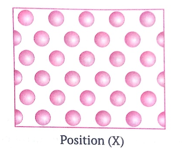
Position (X)
This is because oil is in liquid state.
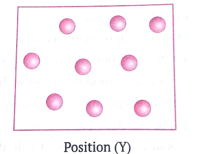
Position (Y)
This is because oil burns to produce gaseous products
Que 2. ‘A small volume of water in a kettle can fill a kitchen with steam’. Explain why.
Ans. The liquid form of water converts into gaseous from in steam.
Its particles move very rapidly in the directions and fill the kitchen as gases completely fills the vessel.
Que 3. A sample of water under study was found to boil at 102oC at normal temperature and pressure. Is the water pure? Will this water freeze at 0
C Comment.
Ans. Its freezing point will be below 0
° C due to the presence od a non-volatile impurity in it.
Que 4. You are given the following substances with their melting and bowling points.
|
Substance |
Melting Point (o C) |
Boiling point (o C) |
|
X |
-219 |
-183 |
|
Y |
119 |
445 |
|
Z |
-15 |
78 |
Identify the physical status of X, Y and Z at room temperature (30°C).
Ans. ‘X’ is gas at room temperature.
‘X’ is solid at room temperature.
‘Z’ is liquid at room temperature.
Que 5.

(a) Name the changes in the terms of processes P, Q, R and S.
(b) Which of the changes are exothermic and endothermic?
Ans. (a) ‘P’ is fusion (melting), ‘Q’ is boiling, ‘R’ is condensation and ‘S’ is sublimation.
(b) ‘P’, ‘Q’ and ‘S’ are endothermic and ‘R’ is exothermic.
Que 6. The temperature-time graph given alongside shows the heating curve for pure wax. From the graph answer the following:
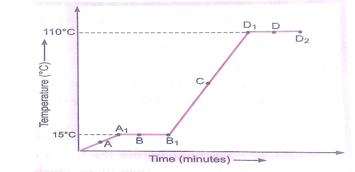
(a) What is the physical state of the substance at the point A, B, C and D?
(b) What is the melting point of the substance?
(c) What is the boiling point?
(d) Which portions of the graph indicates that change to state is taking place?
(e) Name the terms used for heat absorbed during change of states involved in above process.
Ans. (a) A-Solid state, B-Both solid and liquid status,
C- liquid state, D-Both liquid and gaseous states
(b) 15°C (c) 110oC
(d) A1B1 and D1D2 (e) A1B1-Latent heat of fusion
D1D2-Latent heat of vaporisation
Que 7. Water as ice has a cooling effect, whereas water as steam may cause severe burns. Explain these observations.
Ans. In case of ice, the water molecules have low energy while in case of steam the water molecules have high energy. The high energy of water molecules in steam is transformed as heat and may cause burns. On the other hand, in case of ice, the water molecules take energy form the body and thus, give a cooling effect.

