Long Answer Questions - 5 Marks
Q. 1. (i) State the method of determining the valency of an element if its atomic number is given.
(ii) Determine the valency of the following elements, the atomic numbers of which are given in parenthesis:
Chlorine (17), Sulphur (16), Aluminium (13)
Ans. (i) The number of electrons gained, lost or shared to make the octet of electrons (in the outermost shell), gives us directly the combining capacity of the element, that is, the valency.
(ii) Elements Atomic no. Electronic configuration Valency
Chlorine 17 2, 8,7 - 1
Sulphur 16 2, 8, 6 - 2
Aluminium 13 2, 8, 3 + 3
Q. 2. What is the gold foil experiment? Name the scientist who performed this experiment. Write the conclusions and shortcomings of Rutherford's model of atom.
Ans. In 1911, Rutherford performed the gold foil experiment. He bombarded a stream of a-particles on a gold foil, a thin sheet which was 0.00006 cm thick in an evacuated chamber. An a-particle is a positively charged helium ion (He2+). A simplified picture of this experiment is shown in the figure.

In this famous experiment, the following observations were made.
(i) Most of the a-particles passed straight through the foil without any deflection. This concluded that most of the space inside of an atom is empty.
(ii) A few α-particles were deflected through small angle aid few through larger angles. This happened due to positive charge on particles and core (nucleus) of the atom. The heavy positively charged 'core' was named as nucleus.
(iii) The number of a-particles which bounced back was very small. This concluded that the volume of the nucleus is very small in comparison to the total volume of the atom. On the basis of gold foil experiment, Rutherford concluded that an atom consists of nucleus which has positive charge and it is surrounded with electrons which are moving around the nucleus. The number of electrons and protons are equal and the entire mass of the atom is concentrated at its nucleus.
Drawbacks in the Rutherford's model
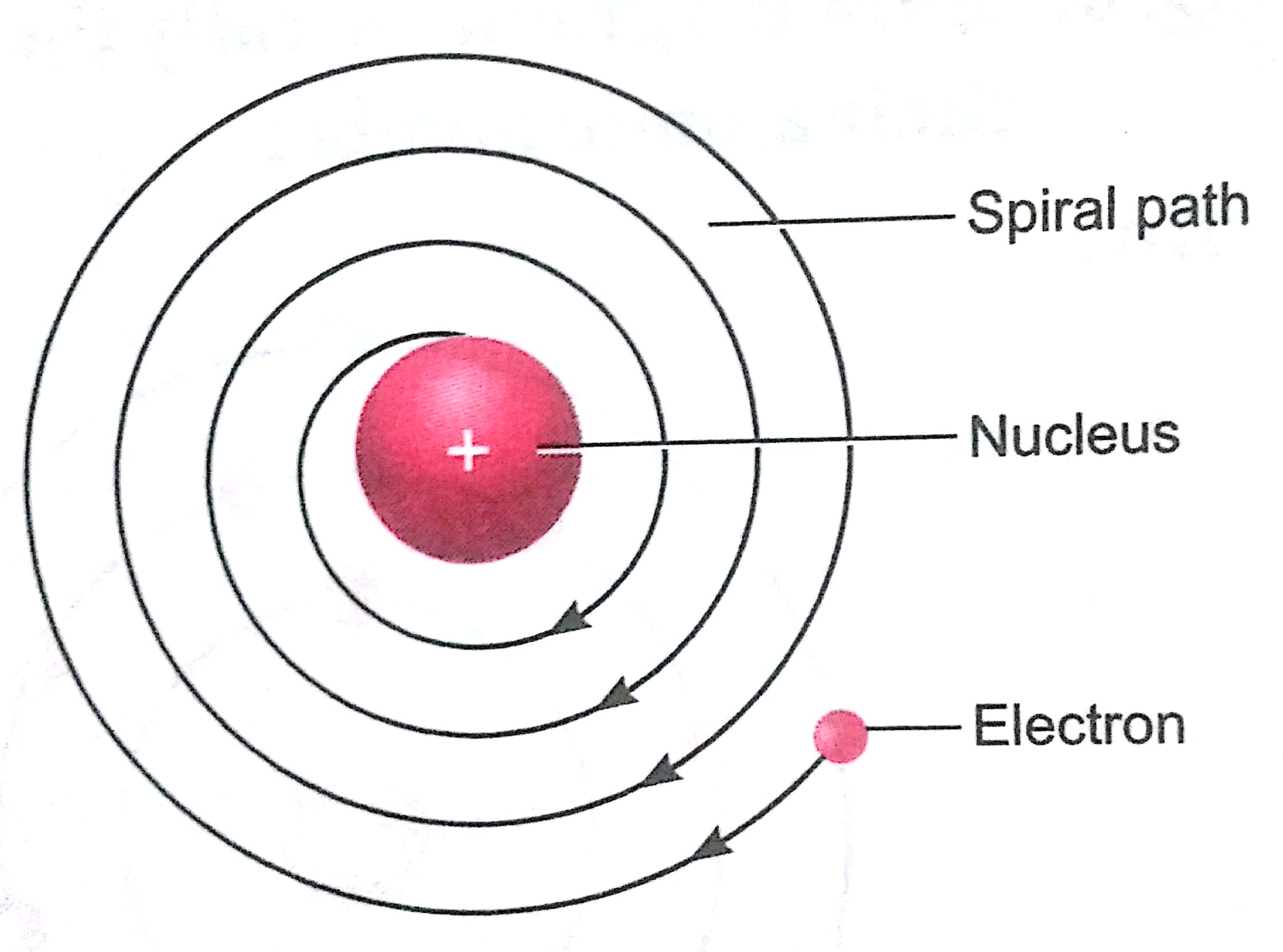
(i) According to classical electro-magnetic theory, a moving charged particle, such as an electron under the influence of attractive force loses energy continuously in the form of radiations. As a result of this, electron should lose energy and therefore, should move in even smaller orbits ultimately falling into the nucleus. But the collapse does not occur. There is no explanation for this behavior.
(ii) Rutherford did not specify the number of orbits and the number of electrons in each orbit.
Q. 3. In what way is the Rutherford's atomic model different from that of Thomson's atomic
model?
Ans. Rutherford proposed a model in which electrons revolve around the nucleus in well-defined orbits. There is a positively charged center in an atom called the nucleus. He also proposed that the size of the nucleus is very small as compared to the size of the atom and nearly all the mass of an atom is centered in the nucleus. Whereas, Thomson proposed the model of an atom to be similar to a Christmas pudding. The electrons are studded like currants in a positively charged sphere like Christmas pudding and the mass of the atom was supposed to be uniformly distributed.
Q.4. What are the postulates of Bohr's model of an atom?
Ans. The postulates put forth by Neil’s Bohr's about the model of an atom:
(a) Only certain special orbits known as discrete orbits of electrons, are allowed inside the atoms.
(b) While revolving in discrete orbits the electrons do not radiate energy. These orbits are called energy levels. Energy levels in an atom are shown by circles.
These orbits are represented by the letters K, L, M, N, .... or the numbers n = 1,2, 3, 4, ….
Q. 5. The ratio of the radii of hydrogen atom and its nucleus is ~105. Assuming the atom and the nucleus to be spherical, (i) what will be the ratio of their sizes? (ii) If atom is represented by planet Earth 'Re’ = 6.4 x 106 m. Estimate the size of the nucleus.
Ans. (i) Volume of the sphere 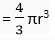
Let R be the radius of the atom and r be that of the nucleus.
Volume of the atom = 
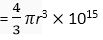
Volume of the nucleus 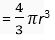
Ratio of the size of atom to that of nucleus = 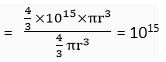
(ii) If the atom is represented by the planet Earth (Re = 6.4 x 106m), then the radius of the nucleus would be


Q.6. Show diagrammatically the electron distribution in a sodium atom and a sodium ion and also give their atomic number.
Ans.

Since the atomic number of sodium atom is 11, it has 11 electrons. A positively charged sodium ion (Na+) is formed by the removal of one electron from a sodium atom. So, a sodium ion has 11-1 = 10 electrons in it. Thus, electron distribution of sodium ion will be 2, 8. The atomic number of an element is equal to the number of protons in its atom. Since, sodium atom and sodium ion contain the same number of protons, therefore, the atomic number of both is 11.
Q. 7. The given figure depicts the atomic structure of an atom of an element 'X’.
Write the following information about the element 'X’.
(a) Atomic number of ‘X’
(b) Atomic mass of ‘X’
(c) Valence electrons
(d) Valency of ‘X’
(e) 'X' should be metal or non-metal.

Ans. (a) Atomic number = Number of protons = 8
(b) Atomic mass = Number of protons + Number of neutrons
= 8 + 10 = 18 u
(c) Valence electrons = 6
(d) Valency of 'X' = 8 – 6 = 2
(e) 'X' should be non-metal because there are six valence electrons hence it will take two more electrons to complete its outermost shell.

