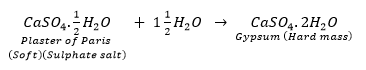Long Answer Questions - 5 Marks
Que 1. (i) In the following schematic diagram for the preparation of hydrogen gas as shown as figure, what would happen if following changes are made?
(a) In place of zinc granules, same amount of zinc dust is taken in the test tube.
(b) Instead of dilute sulphuric acid, dilute hydrochloric acid is taken.
(c) Sodium hydroxide is taken in place of dilute sulphuric acid and the tube is heated.
(ii) How do metal carbonates and metal hydrogencarbonates react will acids?

Ans. (i) (a) Hydrogen gas will evolve with greater speed.
(b) Almost same amount of gas is evolved.
(c) If sodium hydroxide is taken, hydrogen gas will be evolved.
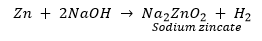
(ii) All metal carbonates and hydrogencarbonates react with acids to form a corresponding salt, carbon dioxide and water.
Metal carbonate + Acid
Metal hydrogencarbonate + Acid
For example, sodium carbonate reacts with dilute hydrochloric acid as follows:
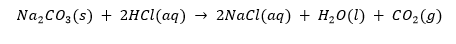
Sodium hydrogencarbonate reacts with dilute hydrochloric acid as follows:
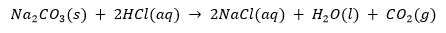
Que 2. A metal carbonates X on reacting with an acid gives a gas which when passed through a solution Y gives the carbonate back. On the other hand, a gas G that is obtained at anode during electrolysis of brine is passed on dry Y, it gives a compound Z, used for disinfecting drinking water. Identify X, Y G and Z.
Ans. The gas evolved at anode during electrolysis of brine is chlorine (G).
When chlorine gas is passed through dry Ca (OH) 2 (Y) produced bleaching powder (Z) used for disinfecting drinking water.
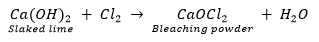
Since Y and Z are calcium salts, therefore X is also a calcium salt and is calcium carbonate.
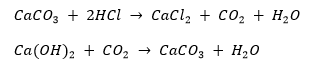
Que 3. Write the formulae of the salts given below.
Potassium sulphate, sodium sulphate calcium sulphate, magnesium sulphate, copper sulphate, sodium chloride sodium nitrate, sodium carbonate and ammonium chloride.
Identify the acids and bases from which the above salts may be obtained. How many families can you identify among these salts?
Ans. The formulae of the given salts and the acids and bases from which these salts may be obtained are given in the following table.
|
S.No. |
Salts |
Formula |
Family |
Acids and Base |
|
1. 2. 3. 4. 5. 6. 7. 8. 9. |
Potassium sulphate Sodium sulphate Calcium sulphate Magnesium sulphate Copper sulphate Sodium chloride Sodium nitrate Sodium carbonate Ammonium chloride |
K2 SO4 NaSO4 CaSO4 MgSO4 CuSO4 NaCl NaNO3 Na2CO3 NH4Cl |
Potassium salts Sodium salts Calcium salts Magnesium salts Copper salts Chloride salts Nitrate salts Carbonate salts Chloride salts |
H2SO4 and K OH H2SO4 and Na OH H2SO4 and Ca (OH)2 H2SO4 and Mg (OH)2 H2SO4 and Cu (OH)2 HCl and NaOH HNO3 and NaOH H2CO3 and NaOH HCl and NH4OH |
Que 4. A sulphate salt of group 2 element of the periodic Table is a white, soft substance, which can be moulded into different shapes by making its dough. When this compound is left in open for some time, it becomes a solid mass and cannot be used for moulding purposes. Identify the sulphate salt and state why does it shows such a behaviour. Give the reaction involved.
Ans. The substance which is used for making different shapes is plaster of Paris. Its chemical name is calcium sulphate hemihydrate (CaSO4. 1/2H2O). The two formula unit of Ca SO4 share one molecule of water. As a result, it is soft.
When it is left open for some time, it absorbs moisture from the atmosphere and forms gypsum, which is a hard solid mass.