Long Answer Questions - 5 Marks
Q. 1. Arrange the following in order of decreasing masses:
(i) 1023 molecules of CO2 gas (ii) 0.1 g atom of silver
(iii) 1 gram of carbon (iv) 0.1 mole of H2SO4
(v) 1023 atoms of calcium.
(Given Atomic masses: Ag = 108 u, S = 32 u, N = 14 u, Ca = 40 u)
Ans. (i) 1 mole of CO2 = 44 g = 6.02 x 1023 molecules
i.e., 6.02 x 1023 molecules of CO2 = 44 g of CO2
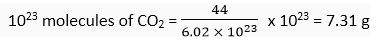
(ii) 1 g atoms of Ag = Gram atomic mass of Ag = 108 g
∴ 0.1 g atom of Ag = 0.1  108 g = 10.8 g
108 g = 10.8 g
(iii) 1 g of carbon = 1g
(iv) 1 mole of H2SO4 = Gram molecular mass
= 2 x 1+ 32 + 4 x 16 = 98 g
∴ 0.I mole of H2SO4 = 0.1 x 98 g = 9.8 g
(v) 1 mole of Ca = 40 g = 6.02  10 23 atoms of Ca
10 23 atoms of Ca
i.e., 6.02 x 1023 atoms of Ca have mass = 40 g

= 6.64 g
Thus, masses in the decreasing order are:
0.1 g atom of Ag > 0.1 mole of H2SO4 > 1023 molecules of CO2 > 1023 atoms of Ca > I g of carbon
Q.2. Calculate the number of aluminium ions (Al3+) in 0.056 g of alumina (Al2O3).
Ans. Molecular mass of alumina (Al203) = 2 × Al3+ + 3 × 02-
= 2 × 27 u + 3 × 16u
= 102 u
Gram molecular mass = 102 g
I mol of alumina (Al203) = 102 g
102 g of Al2O3 = 1 mol
∴ 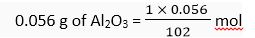
= 5.49 x 10-4 mol
We know that one mol of alumina contains 2 mol of Al3+ ions.
∴ 5.49 x 10-4 mol of Al2O3 contains 2 x 5.49 x 10-4 mol of Al3+ ions
∴ Number of Al3+ ions in 0.056 g = 2 × 5.49 × 10-4 x 6.022 x 1023
= 6.613 x 1020 ions of AI3+
Q. 3. Calculate the mass per cent of each element present in the molecule of calcium carbonate.
Ans. Molecular formula of calcium carbonate = CaCO3
Molecular mass of CaCO3 = 1 × Ca + 1 x C + 3 × 0
= 1 x 40 u + 1 x 12 u + 3 x 16 u = 100 u
Gram molecular mass = 100 g/mol
1 mol of CaCO3 = 100 g
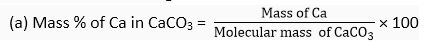
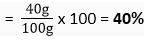
(b) Mass % of carbon in CaCO3 = 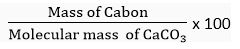

(c) Mass % of oxygen in CaCO3 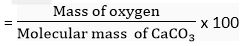
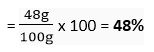
Q. 4. Verify by calculating that
(a) 5 moles of CO2 and 5 moles of H2O do not have the same mass.
(b) 240 g of calcium and 240 g of magnesium elements have a mole ratio of 3 : 5.
Ans. (a) CO2 has molar mass = 44 g mol-1
5 moles of Co2 have molar mass = 44 x 5 = 220 g
H20 has molar mass = 18 g mol-1
5 moles of H2O have mass = 18 × 5 g = 90 g
(b) Number of moles in 240 g Ca metal = 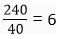
Number of moles in 240 g of Mg metal = 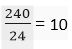
Ratio is 6 : 10
Or, 3 : 5
Q.5. Find the ratio of mass of the combining elements in the following compounds:
(a) CaCO3 (b) MgCl2 (c) H2SO4
(d) C2H5OH (e) NH3 (f) Ca(OH)2
Ans. (a) CaCO3 (b) MgCl2 (c) H2SO4
Ca : C : O × 3 Mg : Cl × 2 H × 2 : S : O × 4
40 : 12 : 16 x 3 24 : 35.5 × 2 1 × 2 : 32 : 16 × 4
40 : 12 : 48 24 : 71 2 : 32 : 64
10 : 3 : 12 1 : 16 : 32
(d) C2H5OH (e) NH3 (f) Ca(OH)2
C × 2 : H × 6 : O N : H × 3 Ca : O × 2 : H × 2
12 × 2 : 1 × 6 : 16 14 : 1 × 3 40 : 16 × 2 : 1 × 2
24 : 6 : 16 14 : 3 40 : 32 : 2
12 : 3 : 8 20 : 16 : 1
Q. 6. Calcium chloride when dissolved in water dissociates into its ions according to the following equation.
CaCl2 (aq) → Ca2+ (aq) + 2Cl- (aq)
Calculate the number of ions obtained from CaCl2 when 222 g of it is dissolved in water.
Ans. I mole of calcium chloride = 111 g
∴ 222 g of CaCl2 is equivalent to 2 moles of CaCl2
Since 1 formula unit CaCl2 gives 3 ions, therefore, 1 mole of CaCl2 will give 3 moles of ions.
2 moles of CaCl2 would give 3 x 2 = 6 moles of ions.
Number of ions = Number of moles of ions x Avogadro number
= 6 × 6.022 × 1023
= 36.132 x 1023
= 3.6132 × 1024 ions.
Q. 7. What is a mole? What is the unit of mole? How many molecules are there in a certain mass of a substance?
Ans. A mole is the amount of a substance which contains the same number of chemical units (atoms,molecules or ions) as there are atoms in exactly 12 g of carbon-12. The unit of mole is given by the symbol 'mol'.
We know that Avogadro number is 6.022 × 1023
Number of molecules in a certain mass
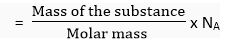
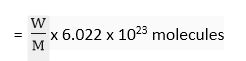
where 'W' is the mass of the substance in which number of molecules is to be calculated and 'M' is the molecular mass of the substance.
Q. 8. The difference in the mass of 100 moles each of sodium atoms and sodium ions is 5.48002 g. Compute the mass of an electron.
Ans. A sodium atom and ion differ by one electron. For 100 moles each of sodium atoms and ions there would be a difference of 100 moles of electrons.
Mass of 100 moles of electrons = 5.48002 g
Mass of 1 mole of electron = 
Mass of one electron =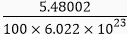
= 9.1 × 10-31 kg
Q.9. The mass of one steel screw is 4.11g. Find the mass of one mole of these steel screws. Compare this value with the mass of the Earth (5.98 × 1024kg). which one of the two is heavier and by how many times?
Ans. 1 mole of steel screws = 6.022 x 1023 screws
Mass of 1 screw = 4.11g
∴ Mass of 1 mole of screws = 4.11 x 6.022 × 1023 g
= 24.75 x 1023 g
= 2.475 x 1024 g
One mole of screw weighs = 2.475 x 1024 g = 2.475 x 1021 kg
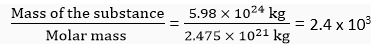
The Earth is 2400 times heavier than one mole of screws.
Q.10. Compute the number of ions present in 5.85 g of sodium chloride.
Ans. 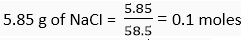
or 0.1 moles of NaCl particle.
Each NaCI particle is equivalent to 2 ions, i.e., one Na+ and one Cl-
Number of ions = 0.2 × 6.022 × 1023
= 1.2042 x 1023 ions
Q. 11. A gold sample contains 90% of gold and the rest copper. How many atoms of gold are present in one gram of this sample of gold?
Ans. One gram of gold sample will contain 
Number of moles of gold = 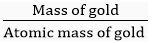
= 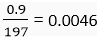
One mole of gold contains NA atoms = 6.022 x 1023
∴ 0.0046 mole of gold will contain = 0.0046 × 6.022 × 1023
= 2.77 x 1021 atoms
Q. 12. Compute the difference in masses of one mole each of aluminium atoms and one mole of its ions. (Mass of an electron is 9.1 x 10-28 g). Which one is heavier?
Ans. Mass of 1 mole of aluminium atom = Molar mass of aluminium = 27 g mol-1.
An aluminium atom needs to lose three electrons to become an ion, Al3+.
For one mole of Al3+ ion, three moles of electrons are to be lost.
The mass of three moles of electrons = 3 x (9.1 x 10-28) x 6.022 x 1023 g
= 27.3 x 6.022 x 10-5g
= 164.400 x 10-5 g = 0.00164 g
Molar mass of Al3+ = (27- 0.00164) g mol-1
= 26.9984 g mol-1
Difference = 27- 26.9984
= 0.0016 g
Q. 13. A silver ornament of mass ‘m’ gram is polished with gold equivalent to 1% of the mass of silver. Compute the ratio of the number of atoms of gold and silver in the ornament.
Ans. Mass of silver = m g

Number of atoms of silver = 
Number of atoms of gold = 
Ratio of number of atoms of gold to silver = Au : Ag
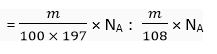
= 108 : 100 x 197
= 108 : 19700
= 1 : 182.41
Q.14. A sample of ethane (C2H6) gas has the same mass as 1.5 x 1020 molecules of methane (CH4). How many C2H6 molecules does the sample of gas contain?
Ans. Mass of 1 molecule of CH4 = 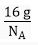
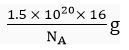
Mass of 1 molecule of C2H6 = 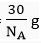
Mass of molecules of C2H6 = 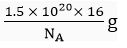
∴ Number of molecules of ethane = 
Q. 15. In photosynthesis, 6 molecules of carbon dioxide combine with an equal number of water molecules through a complex series of reactions to give a molecule of glucose having a molecular formula C6H12O6. How many grams of water would be required to produce 18 g of glucose? Compute the volume of water so consumed assuming the density of water to be 1g cm-3.
Ans.

1 mole of glucose needs 6 moles of water
180 g of glucose needs (6 × 1 8) g of water
Ig of glucose will need  of water.
of water.
18 g of glucose would need 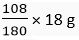 of water = 10.8 g
of water = 10.8 g
Volume of water used = 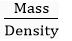
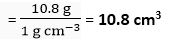
Q. 16. Calculate the ratio between the mass of one atom of hydrogen and mass of one atom of silver.
Ans. 1 mole of H atoms = 1 g
1 mole of H atoms = 6.022 × 1023 atoms.
Mass of 6.022 x 1023 atoms of H = 1g
∴ Mass of one atom of H =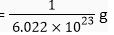
= 1.66 x 10-24 g
1 mole of silver atoms = 108 g
1 mole of silver contains 6.022 × 1023 atoms
∴ 6.022 x 1023 atoms of silver = 108 g
∴ Mass of one atom of silver atom = 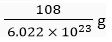
= 1.793 × 10-22g
Ratio between masses of silver and hydrogen atoms
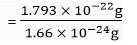
= 1.080 × 102

