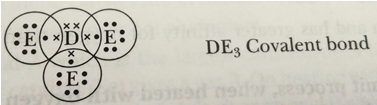Long Answer Questions - 5 Marks
Que 1. What are ionic or electrovalent compounds? Give an example of ionic compound. Explain with reason four properties of these compounds.
Ans. Ionic compounds are those compounds which are formed by the transfer of electrons from a metal to a non-metal. For example, NaCl.
Properties:
(i) Physical nature: Ionic compounds are hard and solid due to strong force of attraction between oppositely charged ions.
(ii) Melting point and boiling point: As more amount of energy is required to break strong bonds. So, they have high melting points and boiling point.
(iii) Solubility: These are soluble in water (polar solvent) but insoluble in organic solvent.
(iv) Conduction of electricity: They conduct electricity in solution or molten state as ions move towards opposite electrodes.
Que 2. Two ores A and B were taken. On heating ore A gives CO2 whereas, ore B gives SO2. What steps will you take to convert them into metals?
Ans. Since ore A gives CO2 and gives SO2. Therefore, ores are MCO3 and MS.
As A is a carbonate ore, it is first subjected to calcination followed by reduction.

As B is a sulphide ore, it is first subjected to roasting followed by reduction.
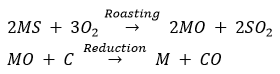
Que 3. Write the names and symbols of two most reactive metals. Explain by drawing electronics structure how any one of them reacts with a halogen. Explain any two physical properties of the compound formed.
Ans. Most reactive metals are Na (sodium) and K (potassium)

Physical Properties:
(i) Physical nature: Hard and solid due to strong attractive forces between oppositely charged ions.
(ii) High melting points and boiling points because more amount of energy is required to break strong force or attraction.
Que 4. (i) Hydrogen is not a metal but it has been assigned a place in the reactivity series of metals. Explain.
(ii) How would you show that silver is chemically less reactive than copper?
Ans. (i) Though hydrogen is not a metal but even then it has been assigned a place in the activity series. The reason is that like metals, hydrogen also has a tendency to lose electron and forms a positive ion H+.
The metals which lose electrons less readily than hydrogen are placed below it and the metals which lose electrons more readily than hydrogen are placed above it in the reactivity series of metals.
(ii) By displacement reaction silver can be shown to be chemically less reactive than copper or copper is more reactive than silver. If a piece of silver is immersed in a solution of copper sulphate, no reaction will take place because silver is less reactive than copper and will not displace copper from the copper from the copper sulphate solution.
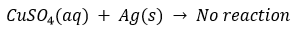
On the other hand, if a copper plate is placed in a solution of silver nitrate, copper will slowly displace silver from the solution and blue solution of copper nitrate is formed.
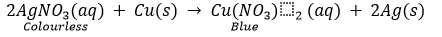
This shows that copper is more reactive than silver.
Que 5. (i) What is an ionic bond?
(ii) How is an ionic bond formed?
(iii) Write the formation of magnesium chloride.
Ans. (i) The chemical bond formed by the transfer of electrons from one atom to another is known as an ionic bond.
(ii) An ionic bond is formed when one of the atoms can donate electrons to achieve the inert gas electronic configuration and other atom needs electrons to achieve the inert gas electronic configuration.
When a metal (usually 1, 2 or 3 electrons in outermost shell) reacts with a non-metal (usually 5, 6 or 7electrons in outermost shell), transfer of electrons takes place from metal atoms to the non-metal atoms and an ionic bond is formed. There is a strong force of electrostatic attraction between metallic cation and non-metallic anion which is responsible for the formation of ionic bond.
(iii) Formation of magnesium chloride (MgCl2): The atomic number of magnesium is 12. It has two electrons in its valence shell as shown below:
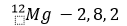
Magnesium, therefore, has a tendency to lose the 2 valence electrons andin the process arraigns the electronic configuration of neon.
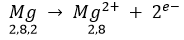
Chlorine (atomic number 17) has 7 electrons in the valence shell. It has a tendency to gain one electron to complete its octet.
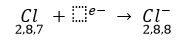
Thus, when magnesium and chlorine are brought together, the magnesium atom transfers its two valence electrons to two chlorine atoms. In the process, both the atoms acquire the stable electronic configuration of nearest inert gases. The positively charged magnesiun ion Mg2+ and negatively charged chloride ions (Cl-) are now held together by the electrostatic force of attraction and form ionic bond.
Mg2+ + 2Cl-
This process can also be shown as below:
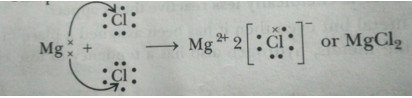
Que 6. (i) Distinguish between ionic and covalent compounds under the following properties:
(a) Strength of forces between constituent elements
(b) Solubility of compounds in water
(c) Electrical conduction in substances
(ii) Explain how the following metals are obtained from their compounds by the reduction process:
(a) Metal M which is in the middle of the reactivity series
(b) Metal N which is high up in the reactivity series
Give one example of each type.
Ans. (i) (a) Ionic compounds have strong force of attraction between the oppositely charged ions (e.g., Na+ and Cl-), so they are solids. Covalent compounds have weak force of attraction between their molecules, so they are usually liquids or gases.
(b) Ionic compounds are soluble in water but covalent compounds are insoluble in water
(c) lonic compounds conduct electricity when dissolved in water or when melted because they contain ions (charged particles). But, covalent compounds like glucose do not conduct electricity because they do not contain ions.
(ii) (a) The metal M which is in the middle of the reactivity series (such as iron, zinc, lead, copper, etc.) is moderately reactive. So, for obtaining such metals from their compounds, their sulphides and carbonates (in which they are present in nature) are first converted into their oxides by the process of roasting and calcination respectively.
For example,
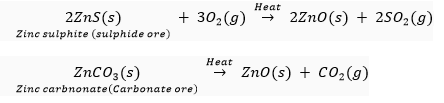
The metal oxides (MO) are then reduced to the corresponding metals by using suitable reducing agents such as carbon. For example, zinc metal from its oxide is obtained as follow:
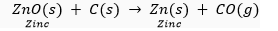
(b) The metal N which is high up in the reactivity series (such as sodium, magnesium, calcium, aluminium, etc.), is very reactive and cannot be obtained from its compound by heating with carbon.
Therefore, such metals are obtained by electrolytic reduction of their molten salt. For example, sodium is obtained by the electrolysis of molten sodium chloride (NaCl).
At cathode: Na++ e-  Na
Na
At anode: 2Cl-  Cl2 + 2e-
Cl2 + 2e-
Que 7. (i) Distinguish between ‘roasting’ and ‘calcination’. Which of these two is used for sulphide ores and why?
(ii) Write a chemical equation to illustrate the use of aluminium for joining cracked railway lines.
(iii) Name the anode, the cathode and the electrolyte used in the electrolytic refining of impure copper.
Ans. (i) Roasting: It is the process in which sulphide ores of the metals are converted into oxides by heating them in the presence of excess air. For example, Zinc sulphide is converted into zinc oxide by roasting.
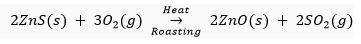
Calcination: It is the process in which carbonate ores of the metals are decomposed into oxides by heating them in the absence or limited air. For example zinc carbonate is decomposed into zinc and carbon dioxide by calcination.
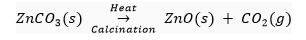
Out of roasting and calcination, only roasting is used for sulphide ores. This is because it is easier to obtain metal from its oxide as compared to its sulphide.
(ii) 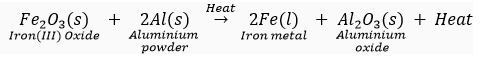
(iii) Anode
Cathode
Electrolyte
Que 8. Write about different chemical processes used for obtaining a metal from its oxides, for metals low in the reactivity series, metals in the middle of reactivity series and metals towards the top of the reactivity series.
Ans. (i) For obtaining the metals that are low in the reactivity series, oxides of such metals can be reduced to metals by simply heating them in the air.
For example, HgS or cinnabar is the ore of mercury metal which on heating changes to HgO.
This metal oxide (HgO) gets reduced to mercury metal (Hg) on further heating.
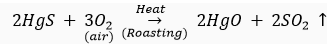
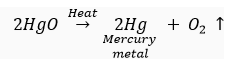
(ii) For obtaining metals that are in the middle of reactivity series, oxides of such metals can be reduced with coke (carbon) which acts as a reducing agent.
For example, iron (III) oxide can be reduced to iron, as follows:
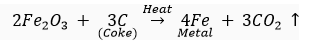
(iii) For obtaining metals that are high up in the reactivity series, their oxides are reduced to metals by electrolysis.
For example,
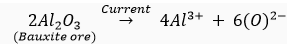
At cathode: 
At anode: 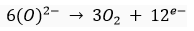
Que 9. (i) How do you classify elements into metals and non-metals on the basis of their electronic configuration? Choose metal and non-metal out of the following:

(ii) What type of bond will be formed if
(a) ‘A ‘combines with ‘B ‘? (b) ‘A’ combines with ‘E’?
(c) ‘C’ combines with ‘E’? (d) ‘D’ combines with ‘E’?
Ans. (i) Elements which contain1 to 3 electrons in their outermost shell are metals.
Elements containing 4 to 7 electrons in their valence shell are non-metals.
Electronic configurations:

Hence A and C are metals whereas, B, D and E are non-metals.
(ii) Type of bonds
(a) ‘A’ is metal and ‘B’ is non-metal, so the bond formed will be ionic.
A = 2, 8, 1 B = 2, 7

(b) ‘A’ is metal and ‘E’ is non-metal, so the bond formed is ionic.
A = 2, 8, 1 E = 2, 8, 7

(c) ‘C’ is metal and ‘E’ is non-metal, so the bond formed is ionic.
C = 2, 8, 2 E = 2, 8, 7
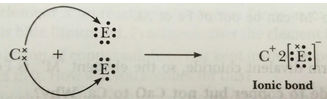
(d) ‘D’ is a non-metal and E is also a non-metal, so the bond formed will be covalent.
D = 2, 8, 5 E = 2, 8, 7