Long Answer Questions - 5 Marks
Que 1. Consider the chemical equation given below and answer the questions that follow:
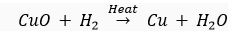
(i) Name the substance which is getting oxidised.
(ii) Name the substance which is getting reduced.
(ii) Name the oxidising agent.
(iv) Name the reducing agent.
(v) What type of a reaction does this equation represent?
Ans. (i) The substance getting oxidised is H2.
(ii) The substance getting reduced is CuO.
(iii) CuO is the oxidising agent.
(iv) H2 is the reducing agent.
(v) since oxidation and reduction is taking place simultaneously, this reaction is an example of redox reaction.
Que 2 Give the Characteristic tests for the following gases:
(i) CO2 (ii) SO2 (iii) O2 (iv) H2
Ans. The Characteristic test for
(i) carbon dioxide (CO2,) gas turns lime water milky when passed through it due to the formation of insoluble calcium carbonate.
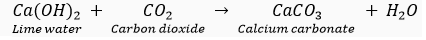
(ii) Sulphur dioxide (SO2) gas when passed through acidic potassium permanganate solution (purple in colour) turns it colourless because SO2 is a strong reducing agent.
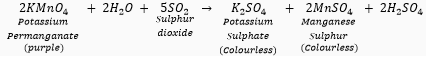
(iii) The evolution of oxygen (O2) gas during a reaction can be confirmed by bringing a burning candle near the mouth of the test tube containing the reaction mixture. The intensity of the flame increases because oxygen supports burning.
(iv) Hydrogen (H2) gas burns with a pop sound when a burning candle is brought near it.
Que 3. With the help of an activity explain that hydrogen and oxygen are released when an electric current is passed through water.
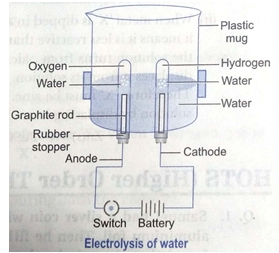
Ans. (i) Take a plastic vessel. Drill two holes at its bottom and set rubber stoppers in these holes.
(ii) Insert carbon electrodes in these rubber stoppers and connect these electrodes to a 6 volt battery and a switch.
(iii) Fill the vessel with water such that the electrodes are immersed. Add a few drops of dilute sulphuric acid to the water in the vessel.
(iv) Take two graduated test tubes filled with water and invert them over the two carbon electrodes.
(v) Switch on the current.
(vi) After sometime, you will observe the formation of bubbles at both the electrodes. These bubbles displace water in the graduated tubes.
(vii) Once the test tubes are filled with the respective gases, remove them carefully.
(viii) Test these gases one by one by bringing a burning splinter of wood close to the mouth of test tubes.
When the glowing splinter of wood is brought close to the mouth of one test tube, it relights and when it is brought close to the mouth of other test tube, the gas burns with a pop. Oxygen is the only common gas that relights the splinter and hydrogen gas burns with a pop.
Que 4. What happens when zinc granules are treated with dilute solution of H2SO4, HCl, HNO3, NaCl and NaOH? Also write the chemical equations if reaction occurs.
Ans. The reaction of Zn granules with
(i) dilute H2SO4
Zn(s) + H2SO4(aq) → ZnSO4(aq) + H2(g)
ZnSO4(aq) + H2(g)
(ii) dilute HCl
Zn(s) + 2HCl(aq) → ZnCl2(aq) + H2(g)
ZnCl2(aq) + H2(g)
(iii) dilute HNO3
Reaction with dilute HNO3 is different as compared to other acids because nitric acid is an oxidising agent and it oxidises h2 gas evolved to H2O
4Zn(s) + 10HNO3(aq) → 4Zn(NO3)2(aq) + 5H2O(l) + N2O(g)
(iv) NaCl solution
Zn(s) + NaCl(aq) → No reaction
(v) NaOH solution
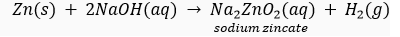
Que 5. (i) Crystals of copper sulphate are heated in a test tube for some time.
(a) What is the colour of copper sulphate crystals before heating, and after heating?
(b) What is the source of liquid droplets seen on the inner upper side of the test tube during the heating process?
(ii) A metal 'X' when dipped in aqueous solution of aluminium sulphate no reaction is observed whereas when it is dipped in an aqueous solution of ferrous sulphate, the pale green solution turns colourless. Identify metal 'X' with reason.
Ans. (i) (a) The colour of copper sulphate crystals before heating heating is blue and turns white after heating.
(b) The liquid droplets are actually the water droplets. The source of water droplets is the water of crystallisation of hydrated copper sulphate crystals (CuSO4.5H2O).
(ii) When metal X' is dipped in aqueous solution of aluminium sulphate no reaction is observed, it means it is less reactive than aluminium. But when it is dipped in ferrous sulphate solution, the solution turns from pale green to colourless, so 'X' is more reactive than iron and thus displaces it from its solution.
Therefore, 'X' must be zinc. It reacts with ferrous sulphate to form colourless: zinc
Solution by displacing iron.
Zn(s) + FeSO4 (aq) → ZnSO4 (aq) + Fe(s)

