Is Matter Around Us Pure
Que 1. (a) Under which category of mixtures will you classify alloys and why?
(b) A solution is always a liquid. Comment.
(c) Can a solution be heterogeneous?
Ans.
(a) Alloys are homogeneous mixture of two or more elements because the constituent elements mix together and give a mixture which uniform together
(b) No, solid solutions and gaseous solutions are also possible. Examples are brass, air.
(c) No, a solution cannot be heterogeneous in nature.
Que 2. The teacher instructed three students ‘A’ ‘B’ and ‘C’ respectively to prepare a 50% (mass by volume) solution of sodium hydroxide (NaOH). ‘A’ dissolved 50 g of NaOH in 100 mL of water to make 100 mL of solution. Which one of them has made the desired solution and why?
Ans. ‘C’ has made the desired solution because 50% (mass by volume) solution means 50 g of solute dissolved in 100 mL of solution.
Mass by volume per cent = 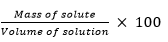
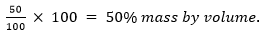
Que 3. Explain why filter paper cannot be used to separate colloids.
Ans. The size of colloidal particles is less then 1nm, while the size of pores present in an ordinary filter paper is large than 1nm. Hence, a colloidal solution cannot be separated by filtration.
Que 4. There student A, B and C prepared mixture using chalk powder, common salt and milk respectively in water. Whose mixture:
(i) would not leave reside on filter paper after filtration?
(ii) would show Tyndall effect?
(iii) would give transparent/clear solution?
(iv) would settle down at the bottom when left undisturbed?
(v) could be filtered by filter paper?
Ans. (i) (a) Mixture of common salt and water.
(b) Mixture of milk and water.
(ii) Mixture of chalk powder with water and milk with water
(iii) Mixture of common salt and water.
(iv) Mixture of chalk powder and water.
(v) Mixture of chalk powder and water.
Que 5. Can we separate alcohol dissolved in water by using a separating funnel? If yes, then describe the procedure. If not, explain.
Ans. We cannot separate alcohol dissolved in water by using separating funnel because both the components are highly soluble in each other. They can be separate by fractional distillation.
Que 6. What is the reason foe running cold water through condenser form lower side to upper side in distillation process?
Ans. In distillation process condensation of vapors take place. To absorb more heat, cold water is passed from lower side so that it will stay for longer time and absorb more heat from the vapors to from liquid state of the substance.
Que 7. You are given two samples of water labeled as ‘A’ and ‘B’. Sample ‘A’ boils at 100 C and sample ‘B’ boils at 102oC. Which sample of water will not freeze at 0
C and sample ‘B’ boils at 102oC. Which sample of water will not freeze at 0 c? Comment.
c? Comment.
Ans. Sample ‘B’ will not freeze at 0 C because it is not pure water. At a atm, the boiling of pure water is 100
C because it is not pure water. At a atm, the boiling of pure water is 100 C and the freezing point of pure water is 0
C and the freezing point of pure water is 0 C.
C.

