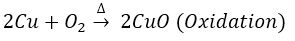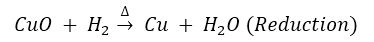Chemical Reactions & Equations
Que 1. Samuel had a Silver coin which turned black. He kept coin in a bowl lined with aluminium foil. Then he filled the bowl with water and boiled it. After sometime, he found that the coin has become new. Its blackness disappeared. How did it happen?
Ans. The blackness of silver coin is due to the formation of silver sulphide in its surface due to its exposure to air. On boiling, the aluminium foil reacts with the layer of silver sulphide and displaces silver from silver sulphide to from aluminium sulphide and silver. This makes the coin shiny.
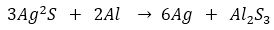
Que 2. During electrolysis of water a few drops of sulphuric acid is added into water. Why?
Ans. Pure water is bad conductor of electricity. By adding a few drops of sulphuric acid, it becomes a good conductor of electricity.
Que 3. Justify with the help of an example that displacement reaction is also a redox reaction.
Ans. Consider the following displacement reaction in which Fe displaces Cu from CuSO4 to form FeSO4:
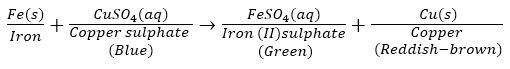
In the above reaction, Fe is gaining oxygen. Hence, Fe is oxidised. CuSO4 is loosing oxygen. Hence, it is reduced. So, it is a redox reaction.
Que 4. Compound ‘A’ when dissolved in water gives compound ‘B’ which used in whitewashing. Compound ‘B’ reacts with CO2 to form a white precipitate of compound ‘C’. Identify compounds ‘A’ ‘B’ and ‘C’. Also write the equations involved.
Ans.
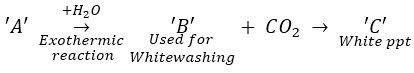
Slaked lime [(Ca (OH) 2] is used for whitewashing. It is obtained when quicklime. CaO reacts with water.
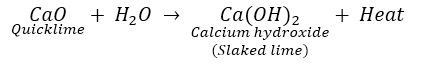
So ‘A’ is CaO and ‘B’ is Ca (OH) 2.
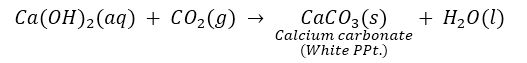
‘C’ is CaCO3.
Que 5. When CaO is added to water taken in a beaker, rise in temperature is observed. However, when Ba (OH) 2 is mixed NH4 Cl, a fall in temperature is observed. Why?
Ans. Reaction of CaO and water is an exothermic reaction. So, rise in temperature is observed.
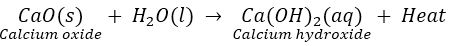
Reaction of Ba (OH) 2 and NH2Cl is an endothermic reaction and hence fall in temperature is observed.
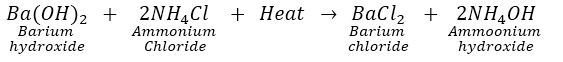
Que 6. A brown substance ‘X’ on heating in air forms a substance ‘Y’. When hydrogen gas is passed over heated ‘Y’, it again changes back into ‘X’.
(i) Name the substance ‘X’ and ‘Y’.
(ii) Name the type of chemical reaction occurring during both the changes.
(iii) Write the chemical equations of the reactions.
Ans. (i) X-Cu; Y-CuO.
(ii) When copper is heated in air, oxidation takes place. When hydrogen gas is passed over heated copper oxide, reduction takes place.
(iii)