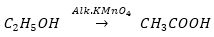Carbon & Its Compound
Que 1. An aldehyde as well as a ketone can be represented by the same molecular formula, say C3H6O. Write their structures and name them. State the relation between the two in the language of science.
Ans.

These two compounds are called isomers i.e., compounds having same molecular formula but different structural formula.
Que 2. An organic acid ‘X’ is a liquid which often freezes during winter time in cold countries, has the molecular formula C2H4O2. On warming it with ethanol is the presence of a few drops of conc. H2SO4, a compound ‘Y’ with a sweet smell is formed.
(i) Identify ‘X’ and ‘y’.
(ii) Write chemical equation for the reaction involved.
Ans. (i) X = Ethanoic acid (CH3COOH)
Y = Ethyl ethanoate (CH3COOC2H5)
(ii) 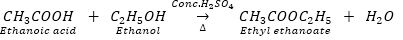
Que 3. Give reason for the following observations:
(a) Air holes of a gas burner have to be adjusted when the heated vessels get blackened by the flame.
(b) Use of synthetic detergents causes pollution of water.
Ans. (a) We need to adjust air holes of gas burner so that sufficient oxygen-rich mixture is burnt to give a clean blue flame for complete combustion.
(b) Synthetic detergents are generally non-biodegradable, that is, they are not decomposed by microorganisms like bacteria. Hence, use of synthetic detergents causes water pollution in lakes and rivers.
Que 4. (a) Why do covalent compounds have low melting points and boiling points?
(b) How are carboxylic acids different from mineral acids from ionisation point of view?
Ans. (a) The molecules in covalent compounds are held by weak van der Waal's forces, hence they have low melting points and boiling points as compared to ionic compounds.
(b) Carboxylic acids (like CH3COOH) ionise to a very small extent in solution and give very small amount of H+ ions. Thus, they are weak acids as compared to the mineral acids.
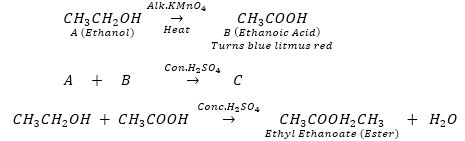
Que 5. An organic compound 'A' is a constituent of wine and beer and is also used as fuel in spirit lamp. Compound 'A' on heating with alkaline potassium permanganate gives another compound 'B' which turns blue litmus to red. Compound ‘A' and 'B' combine in the presence of conc. H2SO4, to give a sweet smelling compound 'C'. Identify compounds ‘A’, 'B' and 'C'. Also write the equations involved in the reaction.
Ans. As compound 'A' is a constituent of wine and beer and is used in spirit lamp it is ethanol.
Que 6. An element of group 14 has two common allotropes, A and B. A is very hard and is bad conductor of electricity while B is soft to touch and good conductor of electricity. Identify the element and its allotropes. Explain reasons for their different properties.
Ans. The element is carbon and the two allotropes are diamond and graphite. Diamond has three-dimensional rigid structure and does not have any free electrons. Hence, it is hard and bad conductor of electricity. Graphite forms hexagonal sheet-like structure and one valency (one electron) with carbon is free. Hence, graphite is soft and a good conductor of electricity.
Que 7. An organic compound A on heating with concentrated H2SO4 forms a compound B which on addition of one mole of hydrogen in presence of Ni forms a compound C. One mole of compound C on combustion forms two moles of CO2 and 3 moles of H20. Identify the compounds A, B and C and write the chemical equations of the reactions involved.
Ans. Since compound C gives 2 moles of CO2 and 3 moles of H20, it shows that it has the molecular formula C2H6 (Ethane). C is obtained by the addition of one mole of hydrogen to compound B so the molecular formula of B should be C2H4 (Ethene). Compound B is obtained by heating compound A with concentrated H2SO4 which shows it to be an alcohol. So compound A could compound A be C2H5OH (Ethanol).
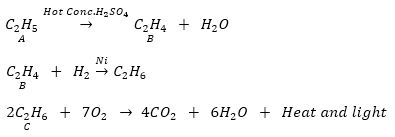
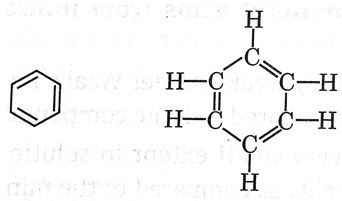
Que 8. A cyclic compound ‘X’ has molecular formula C6H6. It is an unsaturated compound and burns with sooty flame. Identify ‘X’ and write its structural formula.
Ans. X = Benzene/C6H6
Structure
Que 9. The structure formula of an ester is:
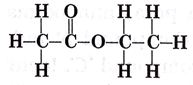
Write the molecular formula of alcohol and acid from which it would have been formed.
Ans. Alcohol
 CH3CH2OH/C2H5OH
CH3CH2OH/C2H5OH
Carboxylic acid  CH3COOH
CH3COOH
Que 10. An organic compounds A of molecular formula C2H6O On heating with excess of Conc. H2SO4 gives compound B of molecular formula C2H4. Compound B on reduction gives compounds C of molecular formula C2H6.
(a) Name A, B and C.
(b) Write chemical equation for the conversion of A to B.
(c) What is the role of Conc. H2SO4 in the above equation?
Ans. (a) A – Ethanol B – Ethene C – Ethane
(b) 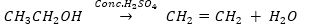
(c) Conc. H2SO4 acts as a dehydrating agent.
Que 11. An organic compounds ‘A’ of molecular formula C2H6O on oxidation with dilute al kal ine KMnO4 solution gives an acid ‘B’ with the same number of carbon atoms. Compound ‘A’ is often used for sterilisation of skin by doctors.
(i) Name the compounds ‘A’ and ‘B’.
(ii) Write the chemical equation involved in the formation of ‘B’ from ‘A’.
Ans. (i) Compound A – Ethanol (ethyl alcohol)
Compound B – Ethanoic acid (acetic acid)
(ii)