Atoms & Molecules
Que 1. A colorless liquid is thought to be a pure compound. Analysis of three samples of the material yield the following results.
|
|
Mass of Sample |
Mass of carbon |
Mass of Hydrogen |
|
Sample 1 |
1.0 g |
0.862 g |
0.138 g |
|
Sample 2 |
1.549 g |
1.335 g |
0.214 g |
|
Sample 3 |
0.988 g |
0.852 g |
0.136 g |
Could the material be a pure compound?
Ans. Analysis
|
|
Mass of Carbon |
+ |
Mass of Hydrogen |
= |
Mass of Sample |
|
Sample 1 |
0.862 g |
+ |
0.138 g |
= |
1.0 g |
|
Sample 2 |
1.335 g |
+ |
2.214 g |
= |
1.549 g |
|
Sample 3 |
0.852 g |
+ |
0.136 g |
= |
0.988 g |
Yes, the material is a pure compound as all the three samples have the same composition.
Que 2. A big drop has volume 1.0 mL. How many molecules of water are there is this drop, If the density of water is 1g/mL?
Ans. Volume of drop of water = 1.0 mL
Density of water = 1.0 g/mL
∴ Mass of drop of water = Volume × Diabesity = 1.0 g
Mass of drop of water = Volume × Diabesity = 1.0 g
Molecular mass of H2O = 2× 1 u + 1 × 16 u = 18 u
Gram molecular mass of water = 18 g/mol
18 g of water contains = 6.022 × 1023 molecular
∴ 1 g of water contains =
1 g of water contains = 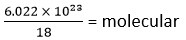
= 
Que 3. What is the fraction of the mass of water due to neutrons?
Ans. Mass of one mole (Avogadro Number) of neutrons ~ 1 g
Mass of the one neutrons = 
Mass of 8 molecule of water = 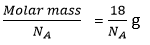
There are 8 neurons of water = 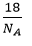
Mass of one molecule of water = 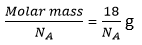
Fraction off mass of the water due to neutrons ~
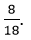
Que 4. You are provided with a fine white coloured powder which is either sugar or salt. How would you identify it without tasting?
Ans. On heating the power, it will char if it is a sugar.
Alternatively, the powder may be dissolved in water and checked for its conduction of electricity. If it conducts, it is a salt.
Que 5. Calculate the number of electrons present in 15.4 of carbon tetrachloride (CCI4 ).
Ans. Number of moles of CCI4 = 
∵ = 0.1 mole
= 0.1 mole
1 mole of CCI4 = 6.022× 1023 molecules of CCI4
∴  0.1 mole of CCI4 = 0.1× 6.022 × 1023 moles of CCI4
0.1 mole of CCI4 = 0.1× 6.022 × 1023 moles of CCI4
= 6.022× 1022 molecules of CCI4
We know that one atom of carbon has 6 electrons and one atom of chlorine has 17 electrons.
Therefore, one molecule of CCI4 will contain 6 + (4× 17) = 74 electrons.
∴  Number of electrons in 6.022× 1022 molecules of CCI4
Number of electrons in 6.022× 1022 molecules of CCI4
= 74× 6.022 ×  1022 electrons
1022 electrons
= 445.6× 1022 electrons
= 4.456× 1024 electrons

