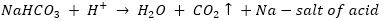Acids,Bases & Salts
Que 1. Why is acetic acid called a weak acid though there are four hydrogen atoms in the molecule?
Ans. Though acetic acid has four hydrogen atoms, only one of the four hydrogen atoms is released as H+ ion in solution. So, it is a weak acid.
Que 2. In one of the industrial processes used for manufacture of sodium hydroxide, a gas X is formed as by-product. The gas X reacts with lime water to give a compound Y which is used as a bleaching agent in chemical industry. Identify X and Y giving the chemical equation of the reactions involved.
Ans. In the manufacture of sodium hydroxide, hydrogen gas and chlorine gas (X) are formed as by-products. When chlorine gas (X) reacts with lime water, it forms calcium oxychloride (bleaching powder) Y. The reactions are
Que 3. A baker found that the cake prepared by him is hard and small in size. Which ingredient had he forgotten to add that would have caused the cake to rise and become light? Give reason.
Ans. The baker had forgotten to add baking powder. Baking powder is a mixture of baking soda (sodium hydrogencarbonate) and a mild edible acid such as tartaric acid. When this baking powder is added with water, then sodium hydrogencarbonate (NaHCO3) reacts with tartaric acid to evolve carbon dioxide gas. This CO2 gas causes the cake to rise and become soft and spongy.
Que 4. A dry pellet of a common base B, when kept in open absorbs moisture and turns sticky. The compound is also a by-product of chlor-al kali process. Identify B. What type of reaction occurs when B is treated with an acidic oxide? Write a balanced chemical equation for one such solution.
Ans. Sodium hydroxide (NaOH) is a commonly used base and is hygroscopic, that is, it absorbs moisture from the atmosphere and becomes sticky.
The acidic oxides react with base to give salt and water. The reaction between NaOH and CO2 can be given as
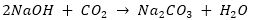
Que 5. A compound 'A' is used in fire extinguishers, as an antacid and its small amount is also used in making bakery items. Identify the compound and also explain the reason for above mentioned uses of the compound 'A'.
Ans. Compound 'A' is sodium hydrogencarbonate-NaHCO3.
(i) It is used in fire extinguishers because it produces CO2 gas on reaction with acid.
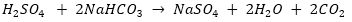
(ii) It is used as an antacid because it neutralises excess acid (HCl) present in stomach.
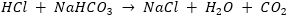
(iii) It is used in making bakery items because on reaction with acid it produces CO2 gas which makes bread, cakes, etc., soft and spongy.